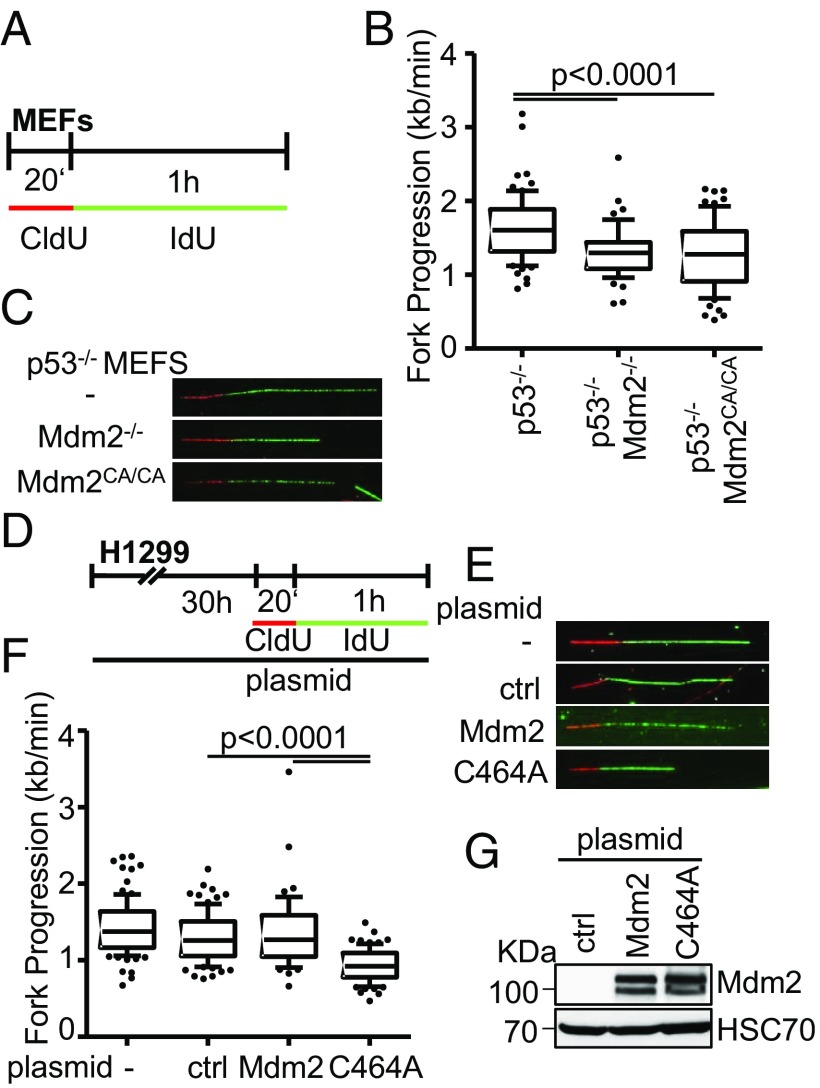
Fig. 4.
The Ring finger domain of Mdm2 supports replication fork progression. (A) Mouse embryonic fibroblasts lacking p53, alone or in combination with a deletion of Mdm2, or a biallelic point mutation in the RING domain of Mdm2 (C462A), were subjected to fiber assay labeling with CldU (20 min) and IdU (60 min). (B) Boxplot analysis of fork progression in the IdU label when either Mdm2 or just its RING domain activity was depleted with 10–90 percentile whiskers. (C) Representative images of the labeled tracks of CldU (red) and IdU (green). (D) H1299 cells were subjected to plasmid transfection for 30 h, followed by DNA fiber assay labeling with CldU for 20 min and IdU for 60 min. (E) Fluorescently labeled tracks of CldU (red) and IdU (green) in untransfected samples as well as upon transfection with the control plasmid pcDNA3 or the expression plasmids pCMV-Mdm2 and pCMV-Mdm2C464A (note that human Mdm2 carries the corresponding cysteine residue that forms part of the Ring finger structure at position 464). (F) Analysis of fork progression in the IdU label shows a significant reduction upon overexpression of RING-mutant Mdm2 but not wild-type Mdm2. (G) Immunoblot analysis upon transfection as in D confirms overexpression of Mdm2 with both a wild-type and a mutant plasmid carrying a point mutation in the RING finger. Two further replicates to the fiber assays in this figure are shown in SI Appendix, Fig. S4.