Abstract
Background
We hypothesized that propofol, a unique general anesthetic that engages N-methyl-D-aspartate and gamma-aminobutyric acid receptors, has antidepressant properties. This open-label trial was designed to collect preliminary data regarding the feasibility, tolerability, and efficacy of deep propofol anesthesia for treatment-resistant depression.
Methods
Ten participants with moderate-to-severe medication-resistant depression (age 18–45 years and otherwise healthy) each received a series of 10 propofol infusions. Propofol was dosed to strongly suppress electroencephalographic activity for 15 minutes. The primary depression outcome was the 24-item Hamilton Depression Rating Scale. Self-rated depression scores were compared with a group of 20 patients who received electroconvulsive therapy.
Results
Propofol treatments were well tolerated by all subjects. No serious adverse events occurred. Montreal Cognitive Assessment scores remained stable. Hamilton scores decreased by a mean of 20 points (range 0–45 points), corresponding to a mean 58% improvement from baseline (range 0–100%). Six of the 10 subjects met the criteria for response (>50% improvement). Self-rated depression improved similarly in the propofol group and electroconvulsive therapy group. Five of the 6 propofol responders remained well for at least 3 months. In posthoc analyses, electroencephalographic measures predicted clinical response to propofol.
Conclusions
These findings demonstrate that high-dose propofol treatment is feasible and well tolerated by individuals with treatment-resistant depression who are otherwise healthy. Propofol may trigger rapid, durable antidepressant effects similar to electroconvulsive therapy but with fewer side effects. Controlled studies are warranted to further evaluate propofol’s antidepressant efficacy and mechanisms of action.
ClinicalTrials.gov: NCT02935647.
Keywords: propofol, depression, NMDA receptor, GABA receptor, electroencephalography
Significance Statement
Treatment-resistant depression afflicts tens of millions of individuals worldwide, causing enormous suffering, economic costs, and mortality. Novel interventions are needed. This study provides the first evidence suggesting that propofol, a widely available anesthetic agent, has rapid and long-lasting antidepressant effects. Future studies are warranted to further evaluate propofol’s antidepressant efficacy and mechanisms of action.
Introduction
Depression is among the most common and debilitating of mental disorders. Although many patients respond to currently available treatments, about one-third have a form of the illness that is not responsive to optimized treatment with antidepressant medications (Rush et al., 2006). Many individuals with severe, treatment-resistant depression pursue electroconvulsive therapy (ECT)—still considered the most effective treatment for depression—but the cognitive side effects of ECT cause many patients to avoid this treatment (Lisanby, 2007). Consequently, each year millions of individuals in the United States alone are debilitated by treatment-resistant depression and left with limited treatment options, at enormous societal costs (Mrazek et al., 2014).
The urgency of this problem has encouraged investigations of novel antidepressant interventions. Agents that target N-methyl-D-aspartate (NMDA) glutamate receptors and gamma-aminobutyric acid (GABA) receptors have appeared particularly promising. Substantial clinical evidence now supports the efficacy of ketamine for treatment-resistant depression (Berman et al., 2000; Zarate et al., 2006; McGirr et al., 2015), and a recent randomized controlled trial demonstrated antidepressant effects of nitrous oxide (Nagele et al., 2015). Several studies have suggested efficacy of another inhaled anesthetic, isoflurane, at high doses in humans (Langer et al., 1985, 1995; Weeks et al., 2013) and rodent models (Antila et al., 2017; Brown et al., 2018). Furthermore, positive GABA-A receptor modulators have shown promising antidepressant effects (Kanes et al., 2017; McMurray et al., 2018). These agents may share pharmacodynamic mechanisms, including inhibition of NMDA receptors and activation of GABAergic neurotransmission, as reviewed recently (Zanos et al., 2018). Indeed, ECT has been reported to reduce NMDA receptor expression and function (Fumagalli et al., 2010; Park et al., 2014), alter glutamatergic synaptic function (Stewart and Reid, 2000; Li et al., 2012), and increase cortical GABA levels in humans (Sanacora et al., 2003). The convergent observations among these diverse interventions suggest a new class of antidepressant agents that rapidly trigger plasticity within glutamate and GABAergic circuitry to induce antidepressant effects (Tadler and Mickey, 2018).
Propofol is a unique, intravenous, general anesthetic that potentiates the function of GABA-A and glycine receptors (Hales and Lambert, 1991) and inhibits the function of NMDA receptors (Orser et al., 1995; Yamakura et al., 1995; Kingston et al., 2006). It has been widely used for over 25 years for procedural sedation and general anesthesia. Propofol is known for its rapid onset and offset of action, tolerability, and safety (Lamperti, 2015). Similar to isoflurane, at high doses propofol induces burst-suppression, a state of intrinsic cortical hyperexcitability that is quantifiable using electroencephalography (EEG) and that is disrupted by blockade of GABA, NMDA, or α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptors (Steriade et al., 1994; Lukatch et al., 2005; Kroeger and Amzica, 2007; Ferron et al., 2009). Taken together, these properties of propofol led us to hypothesize that high-dose propofol would have antidepressant effects and a favorable side-effect profile. To collect preliminary evidence of feasibility, tolerability, and efficacy among individuals with treatment-resistant depression, we performed an open-label trial of deep propofol anesthesia.
Methods
Design and Participants
This open-label study was approved by the University of Utah Institutional Review Board and preregistered at ClinicalTrials.gov (NCT02935647). All participants provided written informed consent. We recruited outpatients who were seen for consultation in a referral clinic for treatment-resistant mood disorders. Assessment included a comprehensive psychiatric evaluation, full medical history, physical examination, screening blood tests (complete blood count, comprehensive metabolic panel, thyroid-stimulating hormone), 12-lead electrocardiogram, and urine pregnancy test as indicated. Inclusion and exclusion criteria (Table 1) were confirmed by a psychiatrist and an anesthesiologist. Importantly, we excluded many individuals with medications or conditions that increased risk of experiencing adverse effects during propofol treatments (e.g., advanced age, severe obesity, hypertension, heart disease). Bipolar depression was not excluded because previous studies of ECT and ketamine have shown similar response rates for bipolar and unipolar depression (Dierckx et al., 2012; Coyle and Laws, 2015; Haq et al., 2015). Of 249 patients screened, 36 met criteria for the study, 11 consented to participate in the study, and 10 received at least 1 treatment. Baseline assessments incorporated the Structured Interview Guide for the Hamilton Depression Rating Scale (HDRS) with Atypical Depression Supplement (Williams et al., 1988) and the Montreal Cognitive Assessment (MoCA) (Nasreddine et al., 2005; Srisurapanont et al., 2017). See supplementary Information for further details about participants.
Table 1.
Inclusion and Exclusion Criteria
| Inclusion |
|---|
| Age 18–55 y, inclusive |
| Primary diagnosis of DSM-5 major depressive disorder or bipolar disorder |
| Current moderate-to-severe depressive episode |
| Minimum of 2 failed antidepressant medication trials of adequate dose and duration (at least one trial within the current depressive episode)a |
| Quick Inventory of Depressive Symptomatology, Self-Rated, total score >10 at baseline |
| 24-item Hamilton Depression Rating Scale total score >18b |
| Exclusion |
| Other current DSM-5 disorders, with the exception of anxiety disorders and attention deficit disorder |
| Electroconvulsive therapy within the past 6 months |
| Lifetime history of DSM-5 cognitive disorder |
| Body mass index >40 |
| Inadequately-treated hypertensionb |
| Daily use of angiotensin converting enzyme inhibitor or angiotensin receptor blocker ** |
| Symptomatic coronary artery disease or congestive heart failureb |
| History of transient ischemic attack or neurologic signs during the past yearb |
| History of or susceptibility to malignant hyperthermiab |
| Any contraindication to propofolb |
| Diabetes requiring insulinb |
| Abnormal kidney functionb |
| Daily use of opioid medicationb |
| Daily use of benzodiazepine medication |
| Pregnant or breastfeeding |
| Psychiatric instability requiring a higher level of care |
| Incompetent to provide consent |
Criteria for propofol participants are listed. Electroconvulsive therapy (ECT) comparison patients were selected based on similar, but not identical, criteria (as noted by a and b).
aECT comparison patients were medication-resistant by clinical history, but antidepressant medication trials were not well documented in all cases.
bCriterion not applied to ECT comparison patients
Treatments
Anesthesiologists administered a series of 10 propofol infusions at a frequency of 3 times per week (1 subject received only 9 treatments due to a holiday schedule conflict). The decision to deliver a series of treatments rather than a single treatment was based on prior experience with ECT, ketamine, and isoflurane, all of which appear to produce higher response rates with 6 to 12 administrations (Langer et al., 1995; Lisanby, 2007; Weeks et al., 2013; Coyle and Laws, 2015). Monitoring included continuous EKG, pulse oximetry, blood pressure by noninvasive cuff, respiratory rate, and end-tidal carbon dioxide. A BIS Monitor (BIS VISTA Monitoring System, Aspect Medical Systems) was applied with a 4-electrode sensor (BIS Quatro, Covidien) to measure the left frontal EEG throughout the procedure. After preoxygenation, the anesthesiologist administered an induction dose of propofol (2,6-diisopropylphenol; Diprivan injectable emulsion; Fresenius Kabi) i.v., started a continuous infusion, and gave additional small boluses as needed. (Propofol dosing is described below.) A laryngeal mask airway and mechanical ventilation were employed. Trendelenburg positioning, IV fluids, and small boluses of pressors were used as needed for hypotension. During the recovery phase, a nurse monitored the participant in a postanesthesia care unit until discharge criteria were met. Further details about treatments are provided in supplementary Information.
Propofol Dosing
Because brain concentrations and pharmacodynamic effects of a given dose of propofol vary substantially between individuals (Ludbrook et al., 2002), propofol dosing was guided by real-time EEG feedback via the BIS Monitor. This approach enabled us to produce relatively consistent pharmacodynamic effects across participants and across treatment sessions. Propofol induction (200–600 mg) was followed by a continuous infusion (300–650 µg/kg/min) and augmented with repeated small boluses (50–100 mg) as needed. Lower induction doses of 200 to 400 mg were used during each subject’s initial treatment to assure hemodynamic stability, and higher induction doses were introduced during later treatments as tolerated. After induction, the infusion rate was adjusted, and additional boluses were given with the goal of maintaining a burst-suppression state with a suppression ratio (SR) of 80% to 100% for 15 minutes. The SR is a metric calculated by the BIS Monitor that indicates the fraction of time the EEG is completely suppressed (isoelectric) during each 1-minute epoch. The rationale for suppressing EEG activity for 15 minutes was that previous studies of burst suppression using isoflurane anesthesia reported antidepressant effects using a similar protocol (Langer et al., 1995; Weeks et al., 2013).
EEG Analysis
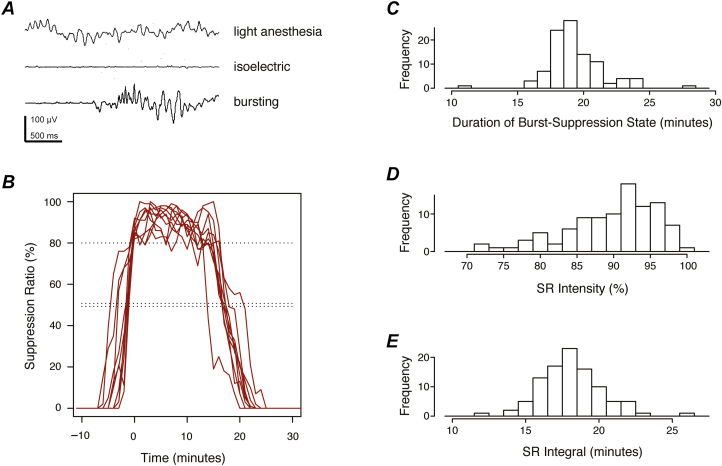
After the procedure, EEG parameters calculated by the BIS Monitor were exported for off-line analysis. As shown in Figure 1, we defined the burst-suppression period of each treatment session as the interval during which SR was >50%. The duration at SR target was defined as the time during which SR was ≥80%. SR intensity was defined as the median SR value during the burst-suppression period. The integral of the SR curve (sum of SR values across all 1-minute epochs during the session) represented the cumulative time spent in the isoelectric state. The average signal quality index calculated by the BIS Monitor exceeded 95% throughout the burst-suppression period of all recording sessions.
Figure 1.
Electroencephalographic (EEG) effects of propofol treatments. (A) Representative EEG traces are shown under light and deep propofol anesthesia. During the burst-suppression state of deep anesthesia, isoelectric periods are occasionally interrupted by stereotypical bursting activity. (B) Suppression ratio (SR) is plotted vs time for a typical participant (10 treatments shown). SR reflects the fraction of time the EEG is isoelectric and free of bursting activity during each 1-minute epoch. The burst-suppression period was defined as the time interval during which SR >50% (double dotted line). Curves are aligned on the time axis such that the first time point at which the target was reached (SR ≥80%, single dotted line) corresponds to 0 minutes. For each treatment session, 3 summary measures were calculated from the SR curve, as shown in histograms to the right. (C) The duration of the burst-suppression state (number of 1-minute epochs during which SR >50%) characterized the “width” of the SR curve. (D) The “height” of the curve was quantified by the SR intensity (median SR value during the burst-suppression period). (E) The integral of the SR curve (sum of SR values across all 1-minute epochs during the treatment session) summarized EEG suppression with a single number that represented total time in the isoelectric state. Histograms show the distributions of these summary measures across all participants and treatments.
Clinical Outcome Measures
The 24-item HDRS (HDRS-24) (Williams et al., 1988; Williams 2001) was administered via a structured interview at baseline, mid-series (48–72 hours after the 5th treatment), and post series (48–72 hours after the final treatment) using a time frame of the most recent 7 days. The MoCA was administered at the same 3 time points (versions 7.1, 7.2, and 7.3, respectively). Self-rated symptoms were collected using the 16-item Quick Inventory of Depressive Symptomatology (QIDS-SR) immediately preceding each treatment and monthly for up to 6 months during the follow-up phase. Duration of unconsciousness was defined as the time from discontinuation of propofol to eyes open. To monitor the acute subjective effects of propofol, the 5-item Drug Effects Questionnaire (Morean et al., 2013) was completed following each treatment session. See supplementary Information for details about clinical outcome measures.
Statistical Analysis
The intended sample size of 10 patients (100 treatments) was chosen to allow us to characterize the feasibility and tolerability of the procedure as well as variation between individuals while adhering to budgetary constraints. Study data were collected and managed using Research Electronic Data Capture hosted at the University of Utah (Harris et al., 2009). All hand-entered data were double checked. Analyses were performed with R statistical software (version 3.2.4) (R Development Core Team, 2008).
The primary, preregistered, depression outcome was HDRS-24 total score, assessed 48 to 72 hours after the final treatment session (3–4 weeks after the first treatment). A positive response was defined a priori as a >50% reduction in the HDRS-24 total score relative to baseline, and remission as a HDRS-24 total score <10. This pilot study was not designed to test the hypothesis that propofol has antidepressant effects, so we report descriptive statistics only, as recommended (Leon et al., 2011). Effect sizes were calculated using the effsize package (version 0.6.2). Posthoc exploratory analyses employed Pearson correlation and linear mixed models, as described in supplementary Information.
Comparison Group
Because the nonspecific effects of the propofol intervention are expected to be very similar to the nonspecific effects of a series of ECT treatments, we gathered outcome data from an ECT cohort as an active comparator arm. A chart review was performed under an institutional review board-granted exemption to collect clinical data from a comparison group of 20 patients who were treated with ECT 3 times per week in the same clinic during the same period as the propofol study. Inclusion and exclusion criteria were similar but not identical to those used for the propofol group (Table 1). Treatments employed standard anesthesia monitoring, methohexital, succinylcholine, and glycopyrrolate. Bifrontal brief-pulse stimulation was used to elicit motor seizures of at least 30 seconds. As with propofol treatments, self-rated symptoms were collected using the QIDS-SR as part of routine clinical care immediately prior to each treatment. Further details about the comparison group are provided in the supplementary Information.
Results
Participants
Baseline demographic and clinical features of the 10 participants are shown in Table 2. All had moderate-to-severe depression despite 2 or more medication trials of adequate dose and duration within the past 2 years, and all were considered good candidates for ECT. Notably, all participants had an onset of illness before age 20 years and a family history of mood disorder (a parent or multiple second-degree relatives), and eight subjects met the criteria for concurrent generalized anxiety disorder. Eight subjects had other medical conditions, all of which were adequately treated.
Table 2.
Baseline Demographic and Clinical Features of the Sample (n=10)
| Demographics | |
|---|---|
| Age, y, mean (SD), range | 33.6 (9.3), 18–45 |
| Sex, female, n | 5 |
| Self-described race, white, n | 10 |
| Marital status, single, n | 5 |
| Married, n | 4 |
| Divorced, n | 1 |
| Education, y, mean (SD), range | 15.5 (2.9), 12–20 |
| Employment status, full-time, n | 7 |
| student, n | 2 |
| unemployed, n | 1 |
| Clinical features | |
| Primary diagnosis, major depressive disorder, recurrent, severe | 9 |
| other specified bipolar disorder a, currently depressed, severe | 1 |
| Right-handed, n | 10 |
| Body mass index, kg/m2, mean (SD), range | 29.3 (5.9), 19.4–37.8 |
| Hamilton Depression Scale, 24-item, mean (SD), range | 34.9 (6.3), 24–45 |
| Hamilton Depression Scale, 17-item, mean (SD), range | 26.0 (4.6), 18–33 |
| Quick Inventory of Depressive Symptomatology SR, mean (SD), range | 19.0 (3.6), 12–25 |
| Montreal Cognitive Assessment, mean (SD), range | 27.7 (1.9), 24–30 |
| Global Assessment of Functioning, mean (SD), range | 45.9 (5.1), 38–55 |
| DSM-5 melancholic features, n | 6 |
| Episode duration, months, mean (SD), range | 17.5 (12.6), 2–42 |
| Onset age, y, mean (SD), range | 13.3 (2.5), 10–19 |
| Number of episodes, median, range | 5.5, 2 to ≥6 |
| Maudsley staging score, mean (SD), range | 8.6 (1.3), 7–11 |
| MGH staging score, mean (SD), range | 5.0 (1.0), 3.5–6.0 |
| Psychotherapy for depression, current, n | 5 |
| past or current, n | 10 |
| Past electroconvulsive therapy, n | 2 |
| Generalized anxiety disorder, n | 8 |
| Social anxiety disorder, n | 3 |
| Panic disorder with agoraphobia, n | 1 |
| Gastroesophageal reflux disease, n | 5 |
| Obstructive sleep apnea, n | 2 |
| Seasonal allergies, n | 2 |
| Tension headaches, n | 1 |
| Asthma, n | 1 |
| Hypothyroidism, n | 1 |
| Acne with dermatitis, n | 1 |
| Supraventricular tachycardia, status post ablation, n | 1 |
No subjects had psychotic or catatonic features; all general medical comorbidities were well controlled.
aDiagnosis of other specified bipolar disorder was based on a distant history of antidepressant-associated, subthreshold manic symptoms.
Feasibility and Acute Treatment Effects
Propofol was dosed with the goal of maintaining the SR at 80% to 100% for 15 minutes. Upon induction with propofol, subjects became unconscious within approximately 1 minute. Approximately 5 minutes after induction, the targeted state of deep anesthesia (SR 80%–100%) was reached, and this state was maintained for 10 to 17.5 minutes (supplementary Table 1). Figure 1 shows a representative example of the EEG effects of this procedure. After propofol was discontinued, participants remained unconscious with eyes closed for 20 to 47 minutes (supplementary Table 2). Once eyes opened, recovery was typically rapid: on average, subjects became fully oriented to time and place 6 minutes after opening their eyes, and they met discharge criteria 10 minutes after eyes open (supplementary Table 2).
Tolerability and Side Effects
Deep propofol anesthesia was well tolerated. Some hypotension was expected given the doses administered, but blood pressure was usually well managed with IV fluids and Trendelenburg positioning. Pressors were typically not needed, but small doses were given occasionally, as used routinely during general anesthesia (supplementary Table 1). During the recovery phase, participants reported neutral or mildly pleasant drug effects on the 5-item Drug Effects Questionnaire (supplementary Table 2). No pressors or other medications were needed during recovery. The most commonly reported side effects were sore throat and discomfort at the IV site; nausea was uncommon (supplementary Table 2). One subject reported double vision, which resolved during recovery after each treatment. No pain was reported. All side effects were described as mild and temporary. No treatment was interrupted due to adverse effects, no participant left the trial early, and no serious adverse events occurred.
Cognitive function did not change substantially during the trial. MoCA total scores improved slightly from 27.7±1.9 (mean±SD) at baseline to 28.9±1.0 at the mid-series time point and 29.1±0.9 at the post-series time point. No participant experienced postprocedure delirium or reported subjective memory problems due to the treatments.
Depression Outcomes
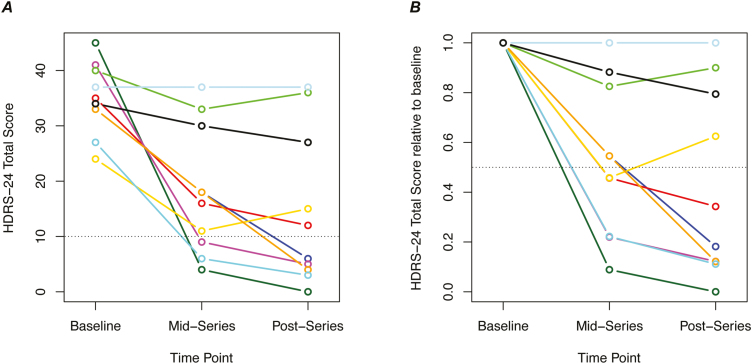
On the primary depression outcome measure, post series HDRS-24, total scores decreased by a mean of 20 points (range 0–45 points), corresponding to a mean 58% improvement from baseline (range 0%–100%). The Hedges’ paired effect size was 1.32 (95% CI: 0.21, 2.42). Six of the 10 subjects met criteria for positive treatment response (>50% improvement) and 5 met criteria for remission (HDRS-24<10) (Figure 2). Much of the improvement in depressive symptoms was evident halfway through the series. After 5 treatments (11–12 days) HDRS-24 had decreased by 17 points on average (range 0–41 points), corresponding to 48% improvement (range 0%–91%), and 3 subjects were in remission.
Figure 2.
Change in depressive symptoms during a series of 10 deep propofol treatments. (A) Total score on the 24-item Hamilton Depression Rating Scale (HDRS-24) is shown at 3 time points during the trial. Each color represents an individual subject. The mid-series assessment was performed 48 to 72 hours after the 5th treatment and the postseries assessment was performed 48 to 72 hours after the final treatment. Scores below the dotted line represent remission of symptoms (HDRS-24<10). (B) HDRS-24 scores in A are re-plotted relative to the individual’s baseline score. Scores below the dotted line represent response (>50% improvement).
Comparison with ECT Patients
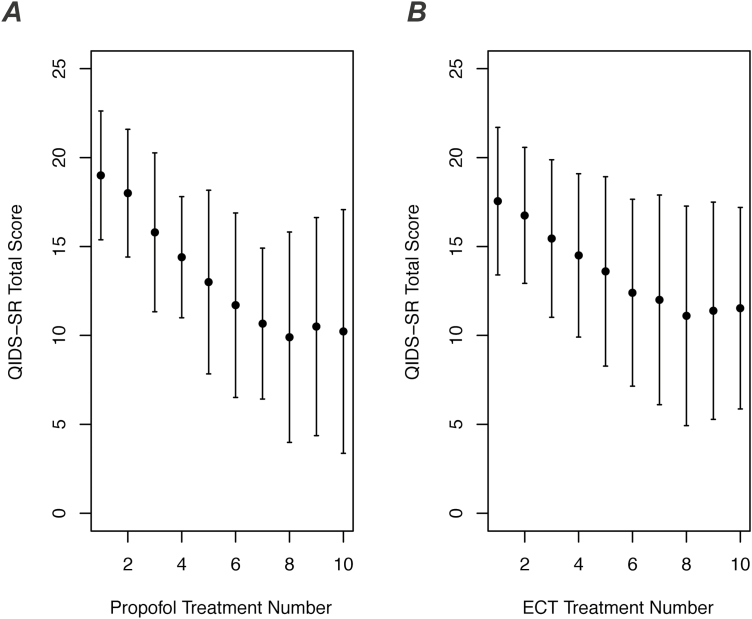
Self-reported depressive symptoms were measured with the QIDS-SR just prior to each propofol treatment session. Because the QIDS-SR was collected from ECT patients in the same way during routine clinical care, we were able to compare depression ratings between propofol participants (n=10) and an ECT comparison group (n=20). The comparison group did not differ significantly from the propofol group with respect to baseline QIDS-SR score (17.6±4.1, mean±SD), age (32.7±9.2 years, mean±SD), sex (50% female), diagnosis (5% bipolar disorder), or body mass index (26.8±5.4, mean±SD) (all P>.25). For both the propofol group and the ECT comparison group, average QIDS-SR scores began in the severe range at treatment 1 and decreased steadily toward the mild-to-moderate range by treatment 10 (Figure 3).
Figure 3.
Change in self-reported depressive symptoms with propofol vs electroconvulsive therapy (ECT). (A) Total score on the self-rated 16-item Quick Inventory of Depressive Symptomatology (QIDS-SR) is shown during a series of 10 propofol treatments (mean±SD, n=10). Subjects completed the QIDS-SR just before each treatment session. (B) QIDS-SR total score is shown for a comparison group of patients who received a series of 10 ECT treatments (mean±SD, n=20).
Follow-up
After propofol treatments were completed, regular contact was maintained with all subjects for at least for 6 months during naturalistic follow-up, during which all patients continued pharmacotherapy. One of the 6 responders relapsed 1 to 2 months after the final propofol treatment; the other 5 remained well for at least 3 months (supplementary Table 3). Of the 4 propofol nonresponders, 3 subsequently pursued ECT and all 3 responded.
Posthoc Analyses
We performed posthoc analyses to test the hypothesis that more intense treatment with propofol would be associated with 3 key outcomes, specifically worse intraoperative hypotension, longer postinfusion recovery, and greater improvement in depression. These exploratory analyses used 2 different measures of treatment intensity as predictors: total propofol dose administered and propofol-induced EEG suppression (as quantified by the SR integral). None of these predictors or outcomes were associated with age or sex (all P>.05).
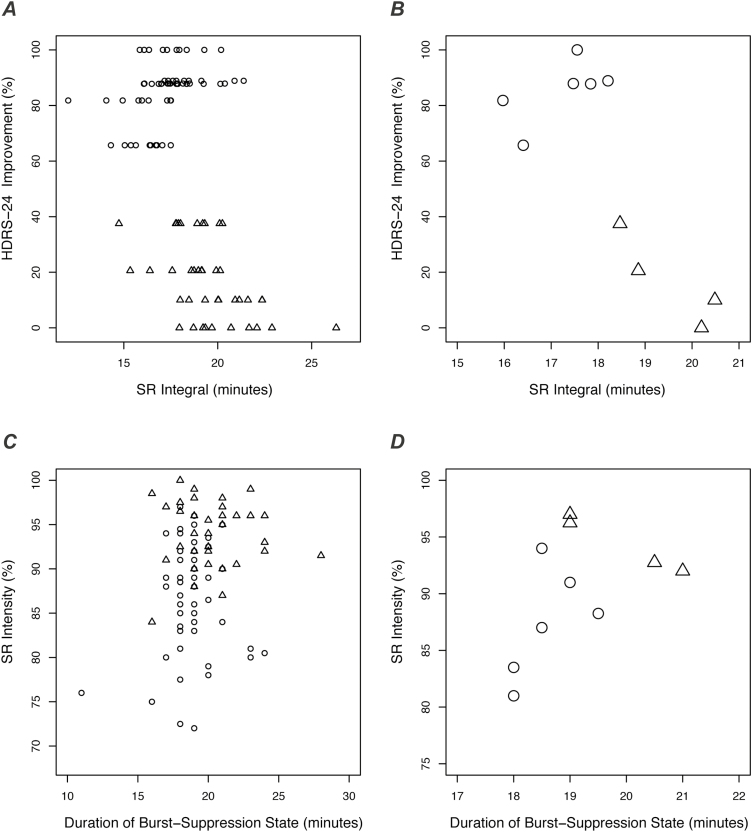
Propofol dose was not significantly associated with any outcome (all P>.10), but analyses did reveal associations with SR integral (i.e., EEG suppression). As hypothesized, greater SR integral predicted worse hypotension at a trend level (χ2=2.6, df=1, P=.10, linear mixed model) and also predicted longer duration of unconsciousness (χ2=22.0, df=1, P=2.7 × 10–6, linear mixed model). Counter to our expectation, SR integral was negatively associated with depression improvement (r=−0.78, P=.0077, n=10, Pearson correlation; Figure 4A,B). In contrast to nonresponders, the treatments of responders were characterized by a duration of burst-suppression <20 minutes and SR intensity <95% (Figure 4C,D). In summary, key outcomes were associated with propofol-induced EEG suppression but not with propofol dose. Lower amounts of EEG suppression predicted less hypotension, quicker recovery, and more improvement in depression.
Figure 4.
Electroencephalographic (EEG) suppression was associated with depression improvement. (A) Improvement in 24-item Hamilton Depression Rating Scale (HDRS-24) is plotted vs suppression ratio (SR) integral for responders (circles) and nonresponders (triangles). SR integral is an EEG measure representing the total time spent in the isoelectric state (explained in Figure 1). Each symbol represents a propofol treatment session. (B) Data from A are re-plotted to show subject-level measures (median values across treatment sessions). (C) As described in Figure 1, SR integral is dependent on both the “height” of the SR curve (SR intensity) and the “width” of the SR curve (duration of burst-suppression state). Here, SR intensity is plotted against duration of burst-suppression for each treatment session and each subject (circles, responders; triangles, nonresponders). (D) Data from C are re-plotted to show subject-level measures (median values across treatments). The treatments of subsequent responders were characterized by duration of burst-suppression <20 minutes and SR intensity <95%.
Discussion
This study is the first to our knowledge to examine the antidepressant effects of propofol. We found that deep propofol anesthesia was feasible and well tolerated in a small sample of physically healthy adults with treatment-resistant depression. This open-label trial also generated preliminary evidence for antidepressant effects, with 6 participants responding after a series of 10 treatments. Improvement of self-reported depressive symptoms among propofol participants was similar to the improvement seen in a matched cohort of ECT patients, and improvements following the propofol intervention typically persisted for months. The cognitive side effects that are typical of ECT were not observed among propofol participants.
Our findings indicate that treatment with high-dose propofol is well tolerated among individuals with treatment-resistant depression who are otherwise in good health. Based on propofol’s known effects on blood pressure in other clinical populations, we expected that pressors would be required routinely and that treatment intensity might be limited by hypotension in some patients, but that was not the case. Hypotension may have been less severe than expected because we did not routinely coadminister propofol with an opioid, as commonly done during surgery. Subjects generally awoke from propofol anesthesia without significant nausea or dysphoria. Self-reports indicated that the drug effects of propofol were typically perceived as neutral or mildly pleasant, consistent with previous reports of low-dose propofol effects in humans and rats (Zacny et al., 1993; Pain et al., 1996). Notably, to optimize safety and tolerability during this pilot trial, we only enrolled individuals age 18 to 55 years, and we excluded concurrent opioid and benzodiazepine medications as well as chronic physical health problems, so tolerability among more typically encountered patients remains unknown. Furthermore, the sample size of our study allows us to only imprecisely estimate the safety of deep propofol anesthesia. Based on the lack of serious adverse events during the 99 treatments administered (one participant received 9 treatments), the frequency of such events is likely less than approximately 1% per treatment session. Finally, addiction and abuse are potential consequences of repeated administration of propofol. Future studies in humans and animal models will be required to fully assess this risk.
We observed no objective or subjective cognitive side effects from propofol beyond the expected drowsiness for several hours following each treatment. Subjects became fully oriented and met postanesthesia discharge criteria approximately 10 minutes after eyes open (approximately 40 minutes after propofol discontinuation). Furthermore, MoCA scores measured during and after the treatment series remained stable in all cases, suggesting that repeated treatments did not cause major cognitive deficits. However, it is important to note that the MoCA is a coarse instrument that is insensitive to mild deficits, and cognitive effects are likely to be subtle among this sample of relatively young, highly educated adults. Future studies incorporating a more extensive neurocognitive battery are needed to determine whether deep propofol anesthesia causes deficits that are mild or confined to specific cognitive domains.
The most important limitation of this study was the open-label trial design. Participants were not randomly assigned to propofol vs a comparison intervention, and neither participants nor research staff were blinded to the intervention. Therefore, although the observed improvement in depression might have been caused by specific effects of propofol, we cannot rule out nonspecific effects. The key nonspecific effects that were not controlled for are: other study-related procedures (e.g., fasting, interactions with research staff, other medications administered); placebo effects (e.g., expectation of improvement); natural history of the disease (i.e., spontaneous fluctuations in symptoms); and statistical regression to the mean. Thus, although the rapid, robust, and durable improvement we observed among the 6 responders was compelling (particularly given this severe treatment-resistant cohort), this open-label study cannot provide definitive proof that propofol has antidepressant effects.
Nonetheless, 3 observations support a specific antidepressant effect for propofol. First, we found that the effects of propofol on self-reported depressive symptoms were similar to the effects of ECT. Because the nonspecific effects of the propofol intervention were very similar to those of the ECT intervention, and ECT is known to be highly effective, the ECT cohort serves as a kind of active control group. Yet, it is important to recognize that the ECT group was selected to be similar to the propofol group and subjects were not randomized to propofol vs ECT, so the 2 groups may have differed systematically in unknown ways, causing bias. Second, we observed that most of those who responded to the propofol intervention experienced lasting effects (not unlike ECT). If the effects of the intervention were nonspecific, we would expect the beneficial effects to wane during the months following the intervention when nonspecific factors are no longer active. Third, our posthoc analyses revealed an association between the degree of EEG suppression during treatments and subsequent improvement in depression (Figure 4). Although the direction of this association was opposite to our prediction, any association would be unexpected if depression improvement were caused by nonspecific effects. In summary, although these results do not allow us to draw definitive conclusions about propofol’s antidepressant effects, we believe the findings are compelling enough to warrant a blinded randomized controlled trial.
The finding that propofol’s EEG effects—and not propofol dose—were associated with clinical outcomes supports the use of EEG as a pharmacodynamic biomarker and argues against rigid mg- or mg/kg-based dosing of propofol. Furthermore, these posthoc analyses suggest that propofol may elicit antidepressant effects only within an optimal range of EEG suppression. As shown in Figure 4D, the treatments of responders were characterized by a median burst-suppression period of 18 to 19.5 minutes and an SR intensity of 80% to 95%, while treatments of nonresponders were longer (>20 minutes) or more intense (>95%). Although caution is warranted when drawing conclusions based on unexpected posthoc findings from such a small sample, these observations raise the possibility that shorter or less intense treatments could be effective. The existence of such a therapeutic window might in part explain the mixed findings from previous studies of isoflurane and sevoflurane (Langer et al., 1985; Greenberg et al., 1987; Carl et al., 1988; Engelhardt et al., 1993; Langer et al., 1995; García-Toro et al., 2001; Weeks et al., 2013). Future studies of propofol should carefully characterize the EEG effects of this intervention.
Our findings may seem at odds with sham-controlled ECT trials indicating that general anesthetics alone lack the robust antidepressant effects of ECT (Rasmussen, 2009), but we believe this older literature is fully consistent with antidepressant effects of high-dose propofol. Notably, to our knowledge, no sham-controlled ECT trial has used propofol in an anesthesia-only comparison group. Furthermore, the propofol doses we used (9–20 mg/kg) were substantially higher than the doses used for procedural sedation (typically 2–3 mg/kg) and produced EEG effects not typically seen during ECT (i.e., a prolonged burst-suppression state). If propofol has true antidepressant effects, those effects are likely to be dose dependent and may require fine-tuning of the induced EEG or neurochemical effects. Nonetheless, others have highlighted the relatively high sham response rates in some of the older literature, raising the possibility that anesthetics beyond ketamine have measurable antidepressant effects when used at lower doses (Rasmussen, 2009; Vutskits, 2018).
In conclusion, deep propofol anesthesia is feasible and well-tolerated among individuals with treatment-resistant depression. Controlled trials in humans and mechanistic studies in animal models are warranted to further examine propofol’s antidepressant effects. If it proves effective, propofol might become a viable therapeutic alternative for many patients with severe depression and limited treatment options. Beyond propofol’s clinical application, dissection of propofol’s mechanisms of action could provide a novel conceptual framework for understanding depression and developing new antidepressant interventions.
Funding
This work was supported by the Department of Psychiatry, University Neuropsychiatric Institute, and Department of Anesthesiology at the University of Utah; the National Institute of Mental Health (K23 MH092648 to BJM); and the Utah Center for Clinical and Translational Sciences (NCATS 8UL1TR000105, formerly UL1RR025764).
Supplementary Material
Acknowledgments
We thank the study participants and their families; Kelly W. Smith for expert advice and assistance with propofol treatments; Lindsey Garcia for help with recruitment; Nathan Pace, Michael Cahalan, Kai Kuck, Jason Huang, and Clayton Anderson for useful discussions; and the nurses, technicians, and administrators of the Treatment Resistant Mood Disorder clinic for their enthusiastic support of this study.
Statement of Interest
None.
References
- Antila H, et al. (2017)Isoflurane produces antidepressant effects and induces trkb signaling in rodents. Sci Rep 7:7811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berman RM, Cappiello A, Anand A, Oren DA, Heninger GR, Charney DS, Krystal JH(2000)Antidepressant effects of ketamine in depressed patients. Biol Psychiatry 47:351–354. [DOI] [PubMed] [Google Scholar]
- Brown PL, Zanos P, Wang L, Elmer GI, Gould TD, Shepard PD(2018)Isoflurane but not halothane prevents and reverses helpless behavior: a role for EEG burst suppression?Int J Neuropsychopharmacol doi: 10.1093/ijnp/pyy029 (Epub ahead of print). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carl C, Engelhardt W, Teichmann G, Fuchs G(1988)Open comparative study with treatment-refractory depressed patients: electroconvulsive therapy–anesthetic therapy with isoflurane (preliminary report). Pharmacopsychiatry 21:432–433. [DOI] [PubMed] [Google Scholar]
- Coyle CM, Laws KR(2015)The use of ketamine as an antidepressant: a systematic review and meta-analysis. Hum Psychopharmacol 30:152–163. [DOI] [PubMed] [Google Scholar]
- Dierckx B, Heijnen WT, van den Broek WW, Birkenhäger TK(2012)Efficacy of electroconvulsive therapy in bipolar versus unipolar major depression: a meta-analysis. Bipolar Disord 14:146–150. [DOI] [PubMed] [Google Scholar]
- Engelhardt W, Carl G, Hartung E(1993)Intra-individual open comparison of burst-suppression-isoflurane-anaesthesia versus electroconvulsive therapy in the treatment of severe depression. Eur J Anaesthesiol 10:113–118. [PubMed] [Google Scholar]
- Ferron JF, Kroeger D, Chever O, Amzica F(2009)Cortical inhibition during burst suppression induced with isoflurane anesthesia. J Neurosci 29:9850–9860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fumagalli F, Pasini M, Sartorius A, Scherer R, Racagni G, Riva MA, Gass P(2010)Repeated electroconvulsive shock (ECS) alters the phosphorylation of glutamate receptor subunits in the rat hippocampus. Int J Neuropsychopharmacol 13:1255–1260. [DOI] [PubMed] [Google Scholar]
- García-Toro M, Segura C, González A, Perelló J, Valdivia J, Salazar R, Tarancón G, Campoamor F, Salva J, De La Fuente L, Romera M(2001)Inefficacy of burst-suppression anesthesia in medication-resistant major depression: a controlled trial. J Ect 17:284–288. [DOI] [PubMed] [Google Scholar]
- Greenberg LB, Gage J, Vitkun S, Fink M(1987)Isoflurane anesthesia therapy: a replacement for ECT in depressive disorders?Convuls Ther 3:269–277. [PubMed] [Google Scholar]
- Hales TG, Lambert JJ(1991)The actions of propofol on inhibitory amino acid receptors of bovine adrenomedullary chromaffin cells and rodent central neurones. Br J Pharmacol 104:619–628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haq AU, Sitzmann AF, Goldman ML, Maixner DF, Mickey BJ(2015)Response of depression to electroconvulsive therapy: a meta-analysis of clinical predictors. J Clin Psychiatry 76:1374–1384. [DOI] [PubMed] [Google Scholar]
- Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG(2009)Research electronic data capture (redcap)–a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform 42:377–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanes S, Colquhoun H, Gunduz-Bruce H, Raines S, Arnold R, Schacterle A, Doherty J, Epperson CN, Deligiannidis KM, Riesenberg R, Hoffmann E, Rubinow D, Jonas J, Paul S, Meltzer-Brody S(2017)Brexanolone (SAGE-547 injection) in post-partum depression: a randomised controlled trial. Lancet 390:480–489. [DOI] [PubMed] [Google Scholar]
- Kingston S, Mao L, Yang L, Arora A, Fibuch EE, Wang JQ(2006)Propofol inhibits phosphorylation of N-methyl-D-aspartate receptor NR1 subunits in neurons. Anesthesiology 104:763–769. [DOI] [PubMed] [Google Scholar]
- Kroeger D, Amzica F(2007)Hypersensitivity of the anesthesia-induced comatose brain. J Neurosci 27:10597–10607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamperti M.(2015)Adult procedural sedation: an update. Curr Opin Anaesthesiol 28:662–667. [DOI] [PubMed] [Google Scholar]
- Langer G, Neumark J, Koinig G, Graf M, Schönbeck G(1985)Rapid psychotherapeutic effects of anesthesia with isoflurane (ES narcotherapy) in treatment-refractory depressed patients. Neuropsychobiology 14:118–120. [DOI] [PubMed] [Google Scholar]
- Langer G, Karazman R, Neumark J, Saletu B, Schönbeck G, Grünberger J, Dittrich R, Petricek W, Hoffmann P, Linzmayer L(1995)Isoflurane narcotherapy in depressive patients refractory to conventional antidepressant drug treatment. A double-blind comparison with electroconvulsive treatment. Neuropsychobiology 31:182–194. [DOI] [PubMed] [Google Scholar]
- Leon AC, Davis LL, Kraemer HC(2011)The role and interpretation of pilot studies in clinical research. J Psychiatr Res 45:626–629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W, Liu L, Liu YY, Luo J, Lin JY, Li X, Wang B, Min S(2012)Effects of electroconvulsive stimulation on long-term potentiation and synaptophysin in the hippocampus of rats with depressive behavior. J Ect 28:111–117. [DOI] [PubMed] [Google Scholar]
- Lisanby SH.(2007)Electroconvulsive therapy for depression. N Engl J Med 357:1939–1945. [DOI] [PubMed] [Google Scholar]
- Ludbrook GL, Visco E, Lam AM(2002)Propofol: relation between brain concentrations, electroencephalogram, middle cerebral artery blood flow velocity, and cerebral oxygen extraction during induction of anesthesia. Anesthesiology 97:1363–1370. [DOI] [PubMed] [Google Scholar]
- Lukatch HS, Kiddoo CE, Maciver MB(2005)Anesthetic-induced burst suppression EEG activity requires glutamate-mediated excitatory synaptic transmission. Cereb Cortex 15:1322–1331. [DOI] [PubMed] [Google Scholar]
- McGirr A, Berlim MT, Bond DJ, Fleck MP, Yatham LN, Lam RW(2015)A systematic review and meta-analysis of randomized, double-blind, placebo-controlled trials of ketamine in the rapid treatment of major depressive episodes. Psychol Med 45:693–704. [DOI] [PubMed] [Google Scholar]
- McMurray KMJ, Ramaker MJ, Barkley-Levenson AM, Sidhu PS, Elkin PK, Reddy MK, Guthrie ML, Cook JM, Rawal VH, Arnold LA, Dulawa SC, Palmer AA(2018)Identification of a novel, fast-acting gabaergic antidepressant. Mol Psychiatry 23:384–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morean ME, de Wit H, King AC, Sofuoglu M, Rueger SY, O’Malley SS(2013)The drug effects questionnaire: psychometric support across three drug types. Psychopharmacology (Berl) 227:177–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mrazek DA, Hornberger JC, Altar CA, Degtiar I(2014)A review of the clinical, economic, and societal burden of treatment-resistant depression: 1996-2013. Psychiatr Serv 65:977–987. [DOI] [PubMed] [Google Scholar]
- Nagele P, Duma A, Kopec M, Gebara MA, Parsoei A, Walker M, Janski A, Panagopoulos VN, Cristancho P, Miller JP, Zorumski CF, Conway CR(2015)Nitrous oxide for treatment-resistant major depression: a proof-of-concept trial. Biol Psychiatry 78:10–18. [DOI] [PubMed] [Google Scholar]
- Nasreddine ZS, Phillips NA, Bédirian V, Charbonneau S, Whitehead V, Collin I, Cummings JL, Chertkow H(2005)The montreal cognitive assessment, moca: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc 53:695–699. [DOI] [PubMed] [Google Scholar]
- Orser BA, Bertlik M, Wang LY, MacDonald JF(1995)Inhibition by propofol (2,6 di-isopropylphenol) of the N-methyl-D-aspartate subtype of glutamate receptor in cultured hippocampal neurones. Br J Pharmacol 116:1761–1768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pain L, Oberling P, Sandner G, Di Scala G(1996)Effect of propofol on affective state as assessed by place conditioning paradigm in rats. Anesthesiology 85:121–128. [DOI] [PubMed] [Google Scholar]
- Park HG, Yu HS, Park S, Ahn YM, Kim YS, Kim SH(2014)Repeated treatment with electroconvulsive seizures induces HDAC2 expression and down-regulation of NMDA receptor-related genes through histone deacetylation in the rat frontal cortex. Int J Neuropsychopharmacol 17:1487–1500. [DOI] [PubMed] [Google Scholar]
- R Development Core Team (2008)R: A language and environment for statistical computing. Vienna: R Foundation for Statistical Computing. [Google Scholar]
- Rasmussen KG.(2009)Sham electroconvulsive therapy studies in depressive illness: a review of the literature and consideration of the placebo phenomenon in electroconvulsive therapy practice. J Ect 25:54–59. [DOI] [PubMed] [Google Scholar]
- Rush AJ, Trivedi MH, Wisniewski SR, Nierenberg AA, Stewart JW, Warden D, Niederehe G, Thase ME, Lavori PW, Lebowitz BD, McGrath PJ, Rosenbaum JF, Sackeim HA, Kupfer DJ, Luther J, Fava M(2006)Acute and longer-term outcomes in depressed outpatients requiring one or several treatment steps: a STAR*D report. Am J Psychiatry 163:1905–1917. [DOI] [PubMed] [Google Scholar]
- Sanacora G, Mason GF, Rothman DL, Hyder F, Ciarcia JJ, Ostroff RB, Berman RM, Krystal JH(2003)Increased cortical GABA concentrations in depressed patients receiving ECT. Am J Psychiatry 160:577–579. [DOI] [PubMed] [Google Scholar]
- Srisurapanont M, Eurviriyanukul K, Suttajit S, Varnado P(2017)Internal consistency and concurrent validity of the montreal cognitive assessment in individuals with major depressive disorder. Psychiatry Res 253:333–337. [DOI] [PubMed] [Google Scholar]
- Steriade M, Amzica F, Contreras D(1994)Cortical and thalamic cellular correlates of electroencephalographic burst-suppression. Electroencephalogr Clin Neurophysiol 90:1–16. [DOI] [PubMed] [Google Scholar]
- Stewart CA, Reid IC(2000)Repeated ECS and fluoxetine administration have equivalent effects on hippocampal synaptic plasticity. Psychopharmacology (Berl) 148:217–223. [DOI] [PubMed] [Google Scholar]
- Tadler SC, Mickey BJ(2018)Emerging evidence for antidepressant actions of anesthetic agents. Curr Opin Anaesthesiol 31:439–445. [DOI] [PubMed] [Google Scholar]
- Vutskits L.(2018)General anesthetics to treat major depressive disorder: clinical relevance and underlying mechanisms. Anesth Analg 126:208–216. [DOI] [PubMed] [Google Scholar]
- Weeks HR 3rd, Tadler SC, Smith KW, Iacob E, Saccoman M, White AT, Landvatter JD, Chelune GJ, Suchy Y, Clark E, Cahalan MK, Bushnell L, Sakata D, Light AR, Light KC(2013)Antidepressant and neurocognitive effects of isoflurane anesthesia versus electroconvulsive therapy in refractory depression. Plos One 8:e69809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams JB.(2001)Standardizing the Hamilton depression rating scale: past, present, and future. Eur Arch Psychiatry Clin Neurosci 251:II6–II12. [DOI] [PubMed] [Google Scholar]
- Williams JBW, Link MJ, Rosenthal NE, Terman M(1988)Structured interview guide for the Hamilton depression rating scale, seasonal affective disorders version (SIGH-SAD). New York: New York State Psychiatric Institute. [Google Scholar]
- Yamakura T, Sakimura K, Shimoji K, Mishina M(1995)Effects of propofol on various AMPA-, kainate- and NMDA-selective glutamate receptor channels expressed in xenopus oocytes. Neurosci Lett 188:187–190. [DOI] [PubMed] [Google Scholar]
- Zacny JP, Lichtor JL, Zaragoza JG, Coalson DW, Uitvlugt AM, Flemming DC, Binstock WB, Cutter T, Apfelbaum JL(1993)Assessing the behavioral effects and abuse potential of propofol bolus injections in healthy volunteers. Drug Alcohol Depend 32:45–57. [DOI] [PubMed] [Google Scholar]
- Zanos P, Thompson SM, Duman RS, Zarate CA Jr, Gould TD(2018)Convergent mechanisms underlying rapid antidepressant action. CNS Drugs 32:197–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarate CA Jr, Singh JB, Carlson PJ, Brutsche NE, Ameli R, Luckenbaugh DA, Charney DS, Manji HK(2006)A randomized trial of an N-methyl-D-aspartate antagonist in treatment-resistant major depression. Arch Gen Psychiatry 63:856–864. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.