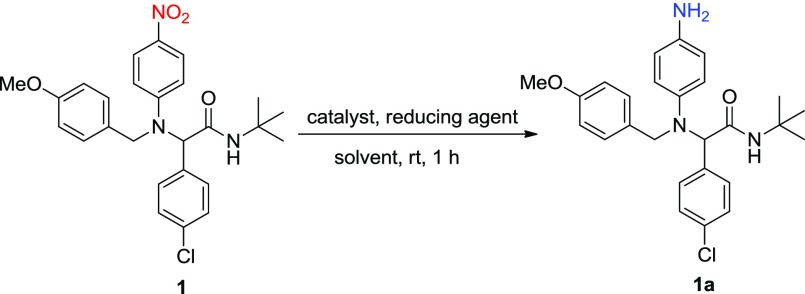
Table 1. Evaluation of Catalysts, Reducing Agents, and Solvents in the Catalytic Reduction of 1 into 1a.
| entry | catalysta | solventa | reducing agenta | time (h) | 1%/1a%b |
|---|---|---|---|---|---|
| 1 | MeOH | NaBH4 | 18 | 100/0 | |
| 2 | THF/MeOH | NaBH4 | 18 | 100/0 | |
| 3c | THF | LiAlH4 | 18 | 43/0 | |
| 4c | THF | NaH | 18 | 47/0 | |
| 5 | THF/MeOH | TMDS | 24 | 100/0 | |
| 6 | Au/TiO2 | THF/MeOH | NaBH4 | 1 | 0/>99 |
| 7 | Au/TiO2 | THF/MeOH | TMDS | 1 | 0/>99 |
| 8 | Au/TiO2 | MeOH | TMDS | 1 | 0/>99 |
| 9 | Ag/TiO2 | THF/MeOH | NaBH4 | 18 | 3/97 |
| 10 | Ag/TiO2 | MeOH | NaBH4 | 18 | 5/95 |
| 11 | Ag/HMS(10) | MeOH | NaBH4 | 18 | 0/>99 |
| 12 | Ag/HMS(10) | THF/MeOH | NaBH4 | 1 | 0/>99 |
| 13d | Ag/HMS(10) | THF/MeOH | NaBH4 | 1 | 60/40 |
| 14 | Ag/HMS(10) | THF/MeOH | TMDS | 1 | 100/0 |
| 15 | Ag/HMS(10) | THF/MeOH | Et3SiH | 18 | 100/0 |
| 16 | Ag/HMS(30) | THF/MeOH | NaBH4 | 1 | 0/>99 |
| 17 | Ag/HMS(50) | THF/MeOH | NaBH4 | 1 | 5/95 |
| 18 | HMS | THF/MeOH | NaBH4 | 1 | 100/0 |
| 19e | AgNO3 | THF/MeOH | NaBH4 | 1 | 2/98 |
| 20e | AgOTf | THF/MeOH | NaBH4 | 1 | 7/93 |
Conditions: 20 mg of the Au/TiO2 or 10 mg of the Ag/TiO2 (ca. 0.8 mol %) or 3 mg of the Ag/HMS (ca. 1.4, 4, and 7 mol %), 0.2 mmol of 1, 0.8 mmol of the hydrosilanes or 0.4 mmol of the sodium borohydride, 1 mL of solvent mixture, at rt.
Relative yields of 1 and 1a at appropriate time measured by 1H NMR of the crude reduction mixture.
Unidentified products missing either the tert-amide or the anisole group from its structure as determined by 1H NMR in some cases.
Equimolar amount of the reducing agent was used.
All the salts were used in 20 mmol %.