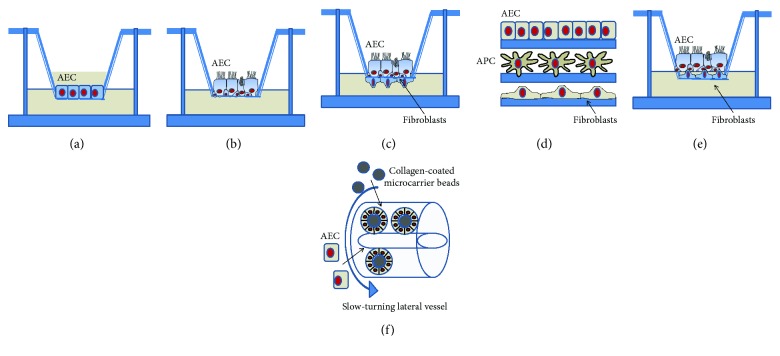
Figure 2.
Models of AEC culture. 2D (a, b, c) and 3D (d, e, f) models are shown. AECs are traditionally cultured onto filter inserts submerged in media until they polarize (a) or differentiate under ALI condition (b). While secondary cell lines polarize but not differentiate at ALI (e.g., Calu-3), primary AECs differentiate into a pseudostratified epithelium presenting ciliated, basal, and mucus-producing goblet cells. These filter inserts can then be removed and upended to allow the attachment of accompanying cell types before the filters are placed back in the well (c). Fibroblasts are shown; however, they could be other cell types, such as DCs or macrophages. An immunocompetent 3D model of the human upper airway was developed using the epithelium, antigen-presenting cells (APC), such as DCs, and fibroblasts (d). Different cell types can individually grow on support and then layered one on top of the other or directly grow in this way. Another 3D model is based on the deposition of human pulmonary fibroblasts onto a collagen-type IV-coated insert, followed by the seeding of AECs and culturing them first in submerged conditions and then at ALI (e). RWV (rotating wall vessel) technology allows the growth of AECs on the surface of porous collagen-coated microcarrier beads in suspension under low fluid shear and gentle mixed conditions inside cylindrical bioreactors, termed slow-turning lateral vessels (STLV) or high aspect ratio vessels (HARV) (f).