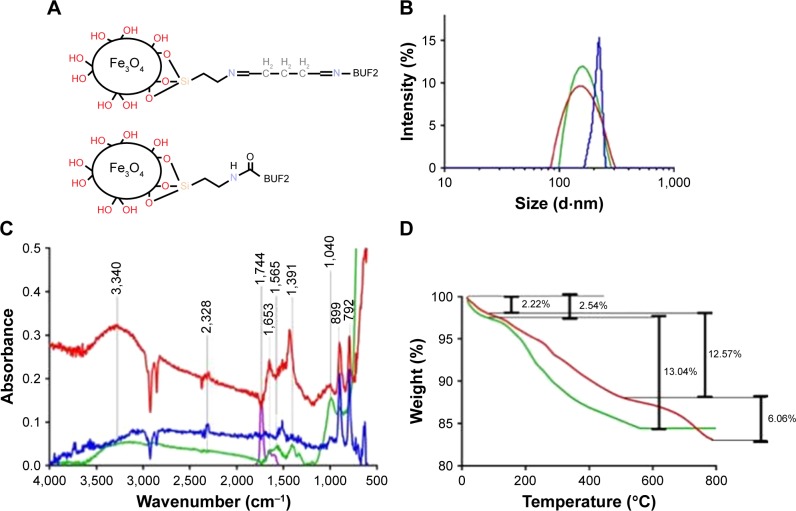
Figure 1.
(A) Schematic of the BUF2-magnetite by conjugation with glutaraldehyde (top) and EDC (bottom) as crosslinkers. (B) DLS histograms for size distribution of magnetite nanoparticles at different functionalization steps. Bare magnetite (red), magnetite functionalized with APTES (green), and magnetite conjugated with BUF2 (blue). (C) FTIR spectra of magnetite (blue), magnetite with APTES (red), BUF2 (purple), and magnetite-APTES-BUF2 (green). (D) TGA of magnetite (green) and magnetite conjugated with BUF2 (red). The first weight loss steps (2.54% and 2.22%) represent the dehydration of the samples. Second weight loss steps (12.57% and 13.04%) correspond to physically absorbed organic solvents. The final weight loss step (6.06%) is attributed to the detachment of BUF2 from the nanoparticle’s surface.
Abbreviations: BUF2, buforin II; EDC, N-[3-(dimethylamino)-propyl]-N′-ethylcarbodiimide hydrochloride; DLS, dynamic light scattering; APTES, (3-aminopropyl) triethoxysilane; FTIR, Fourier transform infrared spectroscopy; TGA, thermogravimetric analysis.