Abstract
STUDY QUESTION
Does the rate of miscarriage increase in the setting of adenomyosis independent of other known risk factors for miscarriage such as maternal age, BMI, embryo genetic status?
SUMMARY ANSWER
Adenomyosis and high BMI both significantly increase miscarriage risk independent of each other, maternal age and embryo health. This study is the first to suggest that ultra-long down regulation GnRH agonist treatment may reduce the rate of early pregnancy loss in adenomyosis patients.
WHAT IS KNOWN ALREADY
The presence of adenomyosis is known to be associated with lower rates of successful implantation and increased risk of early pregnancy loss. However, it is presently unclear whether this reproductive impairment is directly mediated by adenomyosis itself, or indirectly caused by adenomyosis association with known risk factors for miscarriage such as obesity and advancing maternal age/foetal aneuploidy.
STUDY DESIGN, SIZE, DURATION
A retrospective cohort study was undertaken in a private infertility (IVF) clinic examining the outcome for women (n = 345) undergoing the transfer of a genetically screened frozen–thawed embryo between 2012 and 2015.
PARTICIPANTS/MATERIALS, SETTING AND METHOD
A total of 171 women who successfully conceived (positive serum βhCG) following the transfer of a single euploid good morphology frozen–thawed embryo were included in analysis after meeting the inclusion criteria. Only the first conception cycle for each patient was included in the study. Patients with known pre-existing medical risk factors for miscarriage (e.g. thrombophilia, poorly controlled diabetes, coeliac disease, SLE, uterine septum, chromosomal abnormalities) and those women undergoing treatment using donated oocytes and surrogacy were excluded. Patients were then classified as having adenomyosis or not based on a high-quality pelvic ultrasound or MRI. The direct and indirect effects of adenomyosis and BMI on overall miscarriage rate by 12 weeks gestation was then assessed using multivariate logistic regression and mediation analysis. Furthermore, the data were also analysed to elucidate the influence of GnRH ultra-long down-regulation therapy on miscarriage rates.
MAIN RESULTS AND ROLE OF CHANCE
Overall, the adjusted rate of miscarriage was higher in those patients with adenomyosis compared to those without (44.1 vs 15.3%, P < 0.0001), with most of these miscarriages occurring at the early biochemical stage. The rate of miscarriage was especially high in adenomyosis patients not receiving GnRH agonist pre-treatment (82.4%), compared to those patients who did receive GnRH pre-treatment (35.7%, P = 0.0089).
LIMITATIONS, REASONS FOR CAUTION
The study is mainly limited by its small sample size and retrospective design which carries inherent potential for bias (i.e. misclassification and errors due to inadequate clinical notes). The small sample size precluded analysis to distinguish how the extent of adenomyosis disease may modify miscarriage risk (i.e. focal or diffuse disease). Furthermore, the relatively low number of adenomyosis patients not receiving GnRH agonist treatment, plus the non-randomized nature of the decision not to offer such treatment, precludes definitive conclusions on the benefit of GnRH agonist therapy to reduce miscarriage risk.
WIDER IMPLICATIONS OF THE FINDINGS
Considering the significant emotional and financial impact of miscarriage, we suggest screening of all women undergoing IVF treatment for the presence of adenomyosis, with consideration given to ultra-long down regulation GnRH agonist treatment in any woman identified as having adenomyosis. Furthermore, given the persistent and often progressive nature of the disease, adenomyosis should also be considered as a potential uterine cause of recurrent miscarriage. Finally, we hope our study highlights the need for high-quality prospective RCT to be undertaken to provide superior evidence for the potential benefit of GnRH agonist pre-treatment.
STUDY FUNDING/COMPETING INTEREST(S)
K.T. is a practicing IVF gynaecologist and holds a minority stake in the publicly listed company Monash IVF. The other authors declare that they have no conflict of interest. This study was financially supported by Flinders University Medical School.
Keywords: adenomyosis, euploid, GnRH agonist, miscarriage, down-regulation
Introduction
Adenomyosis, the presence of ectopic endometrial tissue within the myometrium, is a well-recognized and common cause of menorrhagia and dysmenorrhoea that traditionally has not been linked with impaired fertility since it is more commonly observed in multiparous women (Brosens et al., 1998). However, a recent meta-analysis of IVF outcomes demonstrated that adenomyosis is associated with lower rates of successful implantation and an increased risk of early pregnancy loss, translating into an overall significant reduction in the live birth rate (Younes and Tulandi, 2017). Since adenomyosis is also associated with advancing maternal age and obesity, two factors known to be associated with impaired pregnancy outcomes (Naftalin et al., 2012; Trabert et al., 2011), it is presently uncertain whether the observed reduction in live-birth potential in adenomyosis patients is a direct result of impaired uterine function, or just an indirect result of increasing rates of maternal age-related embryo aneuploidy and obesity reproductive dysfunction.
WHAT THIS MEANS FOR PATIENTS
This research looks at whether women who have adenomyosis, a condition where the womb lining grows into the muscle wall of the womb, have an increased risk of miscarriage. Adenomyosis is linked to obesity and is more common in older women, both of which can also have an impact on the risk of miscarriage, and in this study the researchers wanted to see whether adenomyosis alone made a difference.
The study looked back at more than 170 women who had a single frozen embryo transferred at a fertility clinic. The women were all screened for adenomyosis and the embryos had all been through genetic screening.
The researchers made sure that any differences in obesity and age were matched in the women in the study, and they found that there was a higher chance of miscarriage with adenomyosis independent of the women’s age or weight. They also found that the risk of miscarriage was reduced when women went through a long down regulation phase of fertility treatment using a drug known as a GnRH agonist.
The researchers suggest that women having IVF should be screened for adenomyosis and if they do have it, a long down regulation phase with a GnRH agonist should be considered, but they acknowledge that further research is needed as this was a small study.
Various lines of evidence suggest impaired uterine function may impede successful live-birth in adenomyosis. First, a precondition for successful pregnancy is a quiescent state of the uterus, with increased uterine contractility being linked with increased subsequent miscarriage risk (Bulletti et al., 1997). Hyper-peristaltic uterine contractions are more commonly seen in women with adenomyosis (Kuijsters et al., 2017), with these contractions even being suggested as an underlying cause of adenomyosis (Leyendecker et al., 2015). Secondly, adenomyosis has been linked with an increase in macrophage and natural killer (NK) cell population density in mid-luteal endometrial biopsies (Tremellen and Russell, 2012), plus an increase in the production of inflammatory mediators and reactive oxygen species (Ota et al., 1998), all posing potential harm to the implanting embryo. Finally, adenomyosis has been associated with altered endometrial steroid hormone production (aromatase activity) and receptor expression (Mehasseb et al., 2011), both key determinants of endometrial functionality.
The primary aim of this study was to investigate whether adenomyosis altered the risk of early pregnancy loss independent of other known risk factors for miscarriage such as maternal age and increasing BMI, plus poor embryo quality. To best achieve this, we analysed only pregnancy outcomes in which good morphology pre-implantation genetic screened euploid embryos were transferred. Subsequently, mediation analysis was used to statistically assess the direct and indirect effects of BMI, age, type of hormonal endometrial preparation and adenomyosis status on the rates of early pregnancy loss.
Materials and Methods
Study cohort
This study was a retrospective cohort analysis of IVF patients, with or without adenomyosis, undergoing genetic screening (PGS) and a frozen embryo transfer between 2012 and 2015 at a private IVF unit (Repromed, Adelaide, Australia). Over this period, it was common practice in the unit to perform genetic screening of embryos in order to maximize viable pregnancy rates from a single embryo transfer, especially in those individuals older than 35 years or who had previous unsuccessful embryo transfers. All patients presenting during this time were considered and consecutively recruited if inclusion and exclusion criteria were satisfied. Inclusion criteria were the presence of a recent (<12-month-old) high-quality pelvic ultrasound and/or MRI to determine adenomyosis status and the subsequent transfer of a solitary euploid embryo in a frozen–thawed cycle. Diagnosis of adenomyosis was performed entirely by ultrasound as all patients undergoing IVF underwent a prior preliminary baseline ultrasound examination. Only the first biochemically confirmed (βhCG positive) pregnancy per patient was considered, and no restriction was made for the number of previously failed cycles. Exclusion criteria included patients with significant pre-existing medical conditions which could independently increase the rate of miscarriage or implantation failure (e.g. thrombophilia, poorly controlled diabetes, Coeliac disease, SLE, uterine septum, polyps, sub-mucosal fibroids, hydrosalpinx, fibroids, chromosomal abnormalities in prospective parents), or those women undergoing treatment using donated oocytes and surrogacy. Women with thyroid disorders were not excluded provided they were euthyroid prior to commencing treatment.
Adenomyosis status
All subjects underwent a screening baseline pelvic morphology ultrasound between Days 2 and 6 of their menstrual cycle using a 9 MHz trans-vaginal probe (GE Health, USA). The diagnosis of adenomyosis was made according to standard radiological criteria (enlarged globular uterus, heterogenous myometrium, sub-endometrial myometrial striations and cysts, asymmetrical thickening of uterine walls, poorly defined junctional zone) (Sakhel and Abuhamad, 2012), with an area of 2 cm or more of adenomyosis being required for a positive diagnosis. In the event of uncertainty, a pelvic MRI was performed to confirm or refute the diagnosis of adenomyosis (junctional zone 12 mm or greater, sub-endometrial cysts). All pelvic MRIs were performed on a Philips Eclipse 1.5 T (Philips, Netherlands) MRI scanner, with a 1.5 T magnet and 27-mT/m gradients in the luteal phase of the menstrual cycle when adenomyosis is most prominent.
IVF treatment protocols
Embryos were generated using ICSI and cultured according to standard protocol as previously reported (Tremellen et al., 2017). Embryos which showed significant compaction on Day 4 or who expanding blastocysts on Days 5–6 with inner cell mass and trophectoderm grading of A or B (Gardner et al., 2000) were deemed suitable for biopsy and cryopreservation.
Whole genome analysis was performed according to manufacturer’s instructions whereby positive (genomic DNA) and negative (amplification mix only) controls were included in each amplification run (SurePlex DNA Amplification System, BlueGnome, Cambridge, UK). Products were processed via comparative genome hybridization (array-CGH technology) (Gutierrez-Mateo et al., 2011).
In ovulatory patients without adenomyosis, embryo transfers were timed after serum hormonal tracking, generally without any form of luteal support. Anovulatory women without adenomyosis were managed either with ovulation induction (clomiphene citrate, letrozole or rFSH), with βhCG luteal support (Pregnyl 1500 IU, MSD, Sydney, Australia), or alternatively with an artificial hormone replacement regime (estradiol valerate 2 mg t.d.s., Progynova, Bayer, Sydney, Australia) for a minimum of 2 weeks, followed by twice-daily vaginal progesterone (Crinone 8%, Merck Serono, Sydney, Australia) once endometrial thickness exceeded 7 mm on ultrasound assessment. Those women identified as having adenomyosis were generally placed in a 4–12-week period of hypo-estrogenic state using long down-regulation GnRH agonist therapy (Zoladex or Lucrin), depending on the severity of the adenomyotic disease and treating physician’s preference. At the end of the down-regulation period endometrial preparation was initiated using either an artificial hormonal regime, as previously outlined, or rFSH ovulation induction followed by βhCG luteal support (Pregnyl 5000 IU trigger, followed by three doses of 1500 IU on Days 4, 7 and 10 of the luteal phase). All subjects receiving long down regulation therapy for adenomyosis were also given low dose prednisolone therapy (15 mg daily) starting a week before ovulation and ending at 11 weeks gestation, as per our previously published protocol (Tremellen and Russell, 2011). Only two of the seven adenomyosis patients not receiving long down-regulation therapy received prednisolone treatment.
Pregnancy was confirmed by serum βhCG assessment 16 days post-ovulation, whereby two rising measurements >5 IU/I indicated a biochemical pregnancy. Clinical pregnancy was defined as the presence of foetal sac at the 6–8 weeks trans-vaginal ultrasound. Biochemical miscarriage was defined as a pregnancy which failed prior to visualization on ultrasound and clinical miscarriage as the presence of a gestational sac with no foetal heart movement. Records of live births,, foetal weight, foetal complications (stillbirths, neonatal deaths and congenital abnormalities) along with maternal complications were recorded as per local legislative requirements.
Ethical review
Ethical review and approval was given by the local institutional Scientific Advisory Committee (Repromed, Dulwich SA, 13/7/16). Written consent for this project was not required as this was a retrospective study and all participants had previously given written approval for their data to be used for retrospective audit studies, as per Australian ethical guidelines (Ethical Considerations in Quality Assurance and Evaluation Activities, 2014).
Statistical analysis
Descriptive statistics were used to describe and compare the demographic and clinical characteristics of participants with or without adenomyosis. Student’s t-test or Mann–Whitney U test was used to compare continuous data, depending on the normal distribution status, and chi-squared or Fischer’s exact test to compare categorical variables. To assess the possible direct and indirect effects of BMI and adenomyosis status on the risk of miscarriage we performed mediation analysis using Mplus software (Muthen & Muthen). Model estimation was performed using maximum likelihood. Due to having a combination of a binary outcome variable (miscarriage) and a normally distributed mediating variable (serum βhCG) we calculated standardized beta coefficients for the indirect, direct and total effects of both BMI and adenomyosis on the risk of miscarriage. The standardized beta coefficients can be interpreted as correlation coefficients that range from −1.0 to +1.0 depending on the strength of the associations between variables. We then assessed the independent effects of adenomyosis status, maternal BMI and log-transformed serum βhCG with additional adjustment for age using multivariate logistic regression with Stata version 14.1 (StataCorp, College Station, TX). Including serum βhCG in the model allowed us to determine the independent effects of BMI and adenomyosis after the effects of βhCG had been removed. In a second model excluding βhCG status, we assessed the differential effects of adenomyosis status on the risk of miscarriage depending on treatment type (natural ovulatory vs long down regulation) by including a treatment type by adenomyosis status interaction in the model, as well as age and BMI. Statistical significance for all tests was determined using two-tailed tests with a Type 1 error rate of alpha=0.05.
Results
Participant characteristics
A total of 345 women underwent PGS in the study period, with 171 women meeting the study inclusion criteria. The remainder were excluded due to no biochemically confirmed (βhCG positive) pregnancy occurring.
The baseline demographic characteristics of the study cohort are outlined in Table I. Notably age, smoking status, gravidity and parity were not significantly different between the adenomyosis negative (n = 137) and adenomyosis positive (n = 34) groups. However, the adenomyosis cohort did have a significantly higher BMI (P = 0.0144), along with an increased duration of infertility (P = 0.0323). As expected, the etiology of infertility was also significantly different between the two groups, with endometriosis being considered the dominant primary etiology of infertility by the treating physician in 23.5% (n = 8) of the adenomyosis positive patients, with male factor infertility (31.4%, n = 43) being the most common diagnosis in the adenomyosis negative control group. Comorbid medical conditions observed in the adenomyosis negative control group include insulin resistance (n = 2), anxiety/depression (n = 9), hypothyroidism (n = 7), epilepsy (n = 2) and Crohn’s disease (n = 1), while in the adenomyosis positive group there was one case each of asthma, anxiety and hypothyroidism.
Table I.
Clinical characteristics of the participants.
| Adenomyosis (n = 34) | No adenomyosis (n = 137) | P-value | |
|---|---|---|---|
| Age (years) | 37.0 ± 4.0 | 35.9 ± 4.6 | 0.2001 |
| (Median 37.3, IQR 32.7–39.4) | (Median 35.6, IQR 34.2–39.5) | ||
| BMI (kg/m2) | 27.6 ± 5.6 | 25.6 ± 6.0 | 0.0144 |
| (Median 26.5, IQR 23.2–31.4) | (Median 23.6, IQR 21.4–28.1) | ||
| Smoking status, n (%) | (n = 30) | (n = 122) | 1.000 |
| Non-smoker | 28 (93.3%) | 113 (92.6%) | |
| Smoker | 2 (6.7%) | 9 (7.4%) | |
| Duration of infertility (months) | 17.5 (IQR 12–36) (Mean 26.8 ± 23.5) | 12, IQR (9.5–24) (Mean 18.6 ± 14) | 0.0323 |
| Etiology | 0.002 | ||
| 1: Male factor | 5 (14.7%) | 43 (31.4%) | |
| 2: Tubal | 3 (8.8%) | 6 (4.4%) | |
| 3: Endometriosis | 8 (23.5%) | 3 (2.2%) | |
| 4: PCOS | 5 (14.7%) | 16 (11.7%) | |
| 5: Advanced maternal age | 1 (2.9%) | 9 (6.6%) | |
| 6: Combined male and female factor | 5 (14.7%) | 25 (18.2%) | |
| 7: Other | 7 (20.5%) | 35 (25.5%) | |
| Gravidity | (n = 33) | (n = 123) | 0.579 |
| 0 | 18 (54.5%) | 70 (56.9%) | |
| 1–3 | 14 (42.4%) | 43 (35%) | |
| ≥4 | 1 (3%) | 10 (8.1%) | |
| Parity | (n = 33) | (n = 123) | 0.347 |
| 0 | 25 (75.8%) | 103 (83.7%) | |
| 1 | 7 (21.2%) | 16 (13%) | |
| ≥2 | 2 (6%) | 4 (3.2%) |
Age and BMI are expressed as mean ±SD, duration of infertility (months) is expressed as median and inter-quartile range. Age was compared with unpaired two-tailed t-test and BMI and duration of infertility with Mann Whitney test. Categorical variables were compared via Chi square test or Fishers Exact as appropriate.
The distribution of the various types of frozen embryo transfer cycle regimens for both the adenomyosis and non-adenomyosis subjects is outlined in Supplementary Table S1. The majority of adenomyosis patients (79.4%, n = 27) underwent ultra-long down regulation therapy followed by either artificial hormone replacement (35.3%, n = 12) or rFSH ovulation induction and βhCG luteal support (44.1%, n = 15). Conversely, in the non-adenomyosis control group the majority (68.6%, n = 94) were naturally ovulatory and simply underwent hormonally timed embryo transfers, while 18.2% (n = 25) required some form of ovulation induction (clomiphene, letrozole or rFSH) or artificial hormone replacement cycle (13.1%, n = 18). Overall, the live birth rate was higher in the non-adenomyosis group (80%, n = 110) compared to adenomyosis group (47%, n = 16) (P = <0.0001) (Supplementary Table SII). Gestational age at birth was not available in the data set, however, average birth weight (3250 ± 742 g vs 3638 ± 575, respectively, P = 0.2728) was not significantly different between the two adenomyosis status groups.
Biochemical data
As evidence of biochemical pregnancy was a criterion for inclusion in this study, we cannot compare implantation rates between the adenomyosis and non-adenomyosis control groups. However, Day 16 post-ovulation serum βhCG levels in the adenomyosis group were significantly lower than those seen in the non-adenomyosis group (478.1 ± 686 vs 591 ± 543 IU/L, respectively; P = 0.0127), while serum progesterone levels were not significantly different (102.5 ± 112 vs 89.1 ± 58, P = 0.1895) (Supplementary Table SI). As obesity is known to lower serum βhCG levels, possibly due to a greater maternal volume of distribution (Gunnala et al., 2017), we performed a regression analysis controlling for the impact of maternal BMI. Importantly, this analysis still confirmed a statistically significant lower βhCG levels in the adenomyosis group at 4 weeks gestation independent of BMI.
The overall (biochemical plus clinical) miscarriage rates were 53% (n = 18) in the adenomyosis group and 19.7% (n = 27) in the non-adenomyosis controls (P = <0.0001), with a 3-fold higher rate of early biochemical miscarriage being observed in the adenomyosis group (44.1 vs 15.3%; P = <0.0006), and a doubling in the clinical miscarriage rate (8.8 vs 4.4%) (Supplementary Table SII).
Mediation analysis
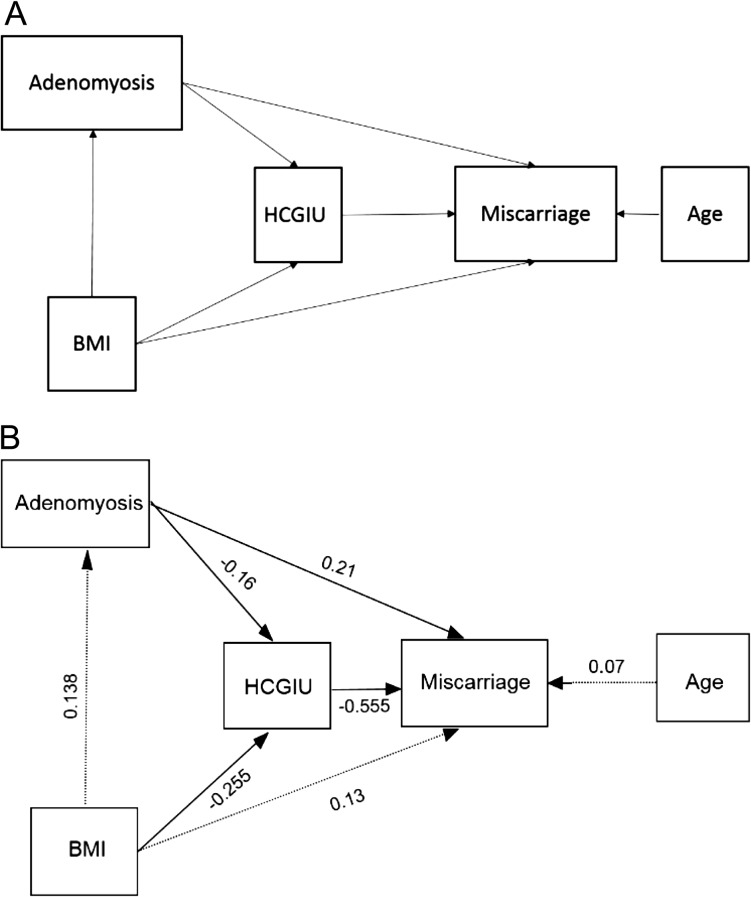
Figure 1 and Supplementary Table SIII show the results for the mediation analysis. There was no significant direct effect of BMI on adenomyosis risk of miscarriage (standardized β = 0.134, P = 0.11). However, there was a significant indirect effect of BMI on the risk of miscarriage mediated via serum βhCG (standardized β = 0.141; SE = 0.044, P = 0.001). The total effect of BMI on the risk of miscarriage was β = 0.317 (P < 0.001). There were a significant direct and indirect effects of adenomyosis status on the risk of miscarriage (standardized β = 0.211; P = 0.03 and β = 0.089; P = 0.033, respectively), with the overall effect of adenomyosis status on the risk of miscarriage also being significant (standardized β = 0.30; P < 0.001).
Figure 1.
Adenomyosis status has a significant direct and indirect effect on the risk of miscarriage. (A) Causal diagram showing hypothesized causal pathways for the direct and indirect effects of BMI and adenomyosis on miscarriage. (B) Standardized effects (®-coefficients) for the path analysis model. Solid lines indicate significant pathways (P < 0.05). ®-coefficients indicate the strength of the correlation between the variables (range −1 to +1).
Logistic regression
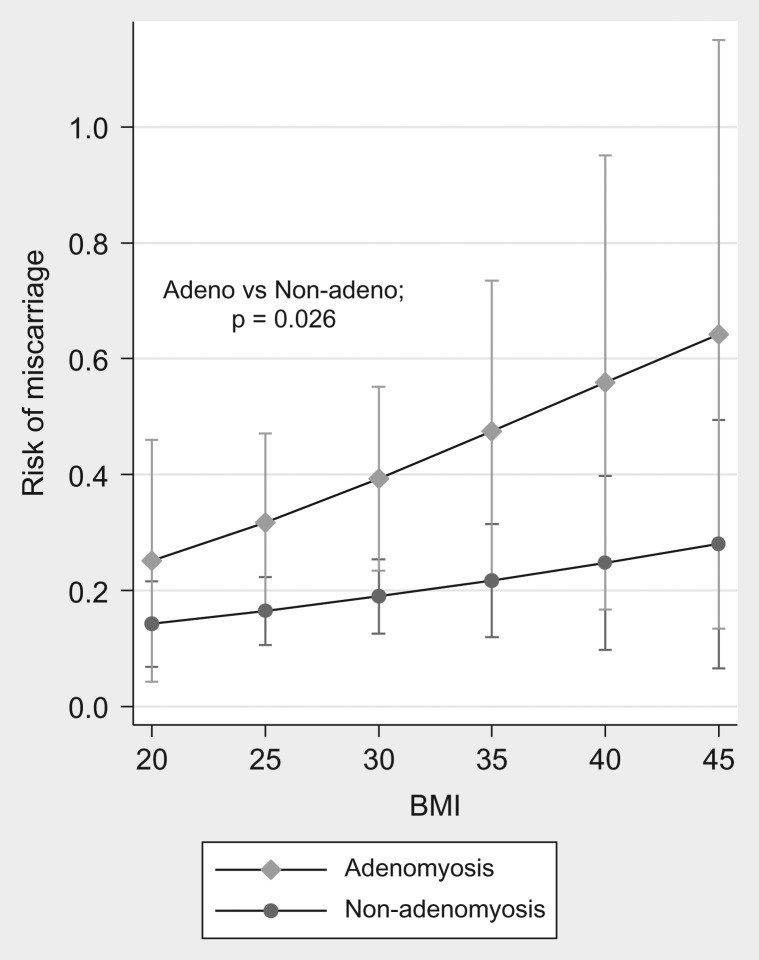
Table II shows the results for the logistic regression models that considered the overall effects of BMI, βhCG, adenomyosis status and treatment modality as predictors of the risk of miscarriage. In the first model that included adenomyosis status, BMI, Day 16 βhCG, and adjustment for age, there was a significant overall effect of adenomyosis (OR = 3.7; 95% CI = 1.4–10.2, P = 0.01), as well as a significant overall effect of log-transformed βhCG on the odds of miscarriage (OR = 0.28; 95% CI = 0.17, 0.47, P < 0.001). There was no significant overall effect of either age or BMI independent of βhCG. Furthermore, there was no significant interaction between adenomyosis status and BMI (P = 0.63) (Fig. 2).
Table II.
Odds ratios for miscarriage showing the independent effects of BMI, βhCG IU and adenomyosis.
| Model 1 | Model 2 | |||
|---|---|---|---|---|
| Odds ratio (95% CI) | P-value | Odds ratio (95% CI) | P-value | |
| Adenomyosis (yes vs no) | 3.74 (1.4, 10.2) | 0.01 | ||
| Age (years) | 1.04 (0.94, 1.15) | 0.47 | ||
| BMI (kg/m2) | 1.06 (0.99, 1.14) | 0.12 | ||
| Log-transformed serum βhCG IU | 0.28 (0.17, 0.47) | <0.001 | ||
| Age (years) | 1.04 (0.95, 1.14) | 0.40 | ||
| BMI (kg/m2) | 1.10 (1.03, 1.17) | 0.005 | ||
| Adenomyosis status/treatment combination | ||||
| Non-adenomyosis—natural | 1.00 | – | ||
| Non-adenomyosis—ultra-long down regulation | 2.3 (0.9, 6.3) | 0.10 | ||
| Adenomyosis—natural | 43.4 (4.5, 420.4) | 0.001 | ||
| Adenomyosis—ultra-long down regulation | 1.92 (0.65, 5.6) | 0.23 |
Figure 2.
Predicted risk of miscarriage as a function of adenomyosis status The (marginal) predicted probabilities were obtained using binary logistic regression with age, BMI, log-transformed HCGUI, adenomyosis and an interaction term for adenomyosis × BMI.
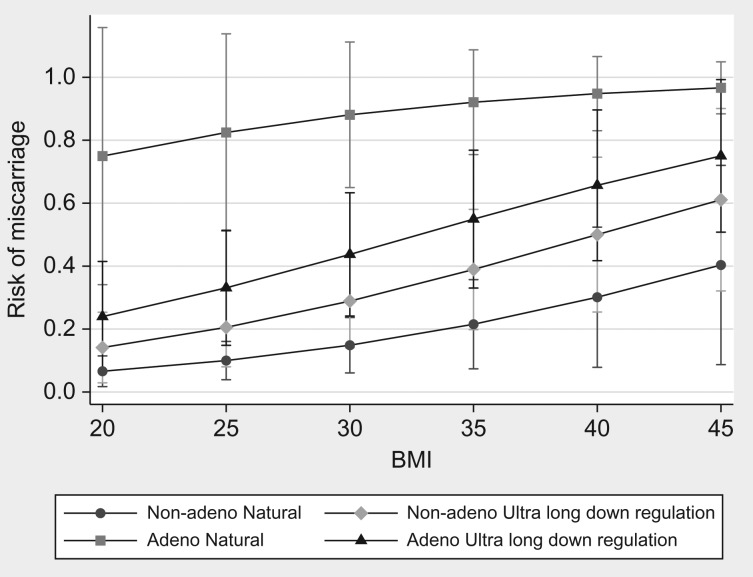
When treatment type and adenomyosis status was included in a logistic regression model as predictors of miscarriage, there was a significant interaction between adenomyosis status and treatment type (P = 0.015) (Fig. 3) indicating that the effects of endometrial preparation (natural, ovulation induction, artificial cycles and ultra-long down regulation) were significantly different depending on adenomyosis status. The risk of miscarriage was significantly higher in women with adenomyosis undergoing natural ovulation cycles, with or without luteal support, compared to those without adenomyosis (82.4 vs 12.0%, P = <0.001). Furthermore, the risk of miscarriage was also higher in adenomyosis patients who did not receive long down regulation treatment compared to those who did (82.4 vs 35.7%, P = 0.0089). However, there was no significant difference in risk of miscarriage for adenomyosis patients undergoing ultra-long down regulation compared to those participants without adenomyosis (35.7 vs 23.2%, P = 0.24).
Figure 3.
Predicted risks of miscarriage as a function of BMI, adenomyosis status and treatment type.
Model 1 shows the direct effects of age, BMI and adenomyosis since the mediating effect of serum βhCG has been removed by inclusion in the model. Model 2 shows the combined impact of treatment and adenomyosis (Table II). Marginal predicted probabilities for adenomyosis and treatment at different levels of BMI were obtained using binary logistic regression with adjustment for age. The effects of adenomyosis were allowed to vary as a function of treatment type by including an adenomyosis by treatment interaction term that was significant (P = 0.015).
Discussion
It is now well recognized that adenomyosis is associated with a significantly lower rate of successful implantation, as attested to by a recent meta-analysis of 11 studies involving over two thousand patients undergoing IVF treatment (Younes and Tulandi, 2017). Our study extends these findings by being the first to show that adenomyosis is also associated with an increased rate of early pregnancy loss, independent of maternal age and embryonic genetic status. While several earlier studies have also reported an increase in miscarriage rate in women with adenomyosis, many did not adequately control for confounders of miscarriage risk such as increasing age and BMI (Younes and Tulandi, 2017). Our study differs by controlling for the impact of maternal age by including only high-quality euploid embryo transfers in the analysis, together with traditional regression analysis—thereby strengthening the direct causal link between adenomyosis and early pregnancy loss. Interestingly, an earlier Spanish study also reported a similar increased risk of miscarriage in adenomyosis patients using embryos created from young oocyte donors (Martinez-Conejero et al., 2011), a legitimate alternative approach of controlling for maternal age-related embryo aneuploidy risk. Importantly, both approaches are consistent with the premise that the adenomyotic uterus provides a dysfunctional environment for the maintenance of pregnancy beyond early implantation events.
In our cohort of patients, those with adenomyosis had a significantly higher BMI than controls, in line with previous literature (Trabert et al., 2011). Since obesity is associated with enhanced estrogen production by adipose aromatase activity (Nelson and Bulun, 2001), impaired ovulation and reduced progesterone (Norman et al., 2004) all potentially drivers for the development of adenomyosis (Taran et al., 2013),this association between increasing BMI and adenomyosis is to be expected. Furthermore, increasing BMI has also been associated with increased risk of euploid miscarriage (Kroon et al., 2011; Boots et al., 2014; Tremellen et al., 2017), possibly mediated by lower serum βhCG driven progesterone production and early pregnancy luteal insufficiency (Shah and Nagarajan, 2013). Therefore, in our statistical analysis, we included maternal BMI as a predictor of miscarriage and assessed both its direct and indirect effects that might be mediated via serum βhCG levels. This analysis clearly demonstrated significant indirect effects of both adenomyosis and BMI, mediated via βhCG. Adenomyosis was associated with reduced βhCG levels early in pregnancy (4 weeks gestation), independent of BMI, and this was associated with increased odds of miscarriage. The embryonic trophoblast is responsible for production of βhCG, with βhCG levels being accepted as an indirect marker of early placental function. The observed reduction in βhCG levels in the adenomyosis cohort suggests that adenomyosis has a significant negative impact on trophoblast function, independent of the transferred embryos initial health (genetic status and morphology) and the woman’s BMI, within just 10 days of implantation.
Miscarriage rates were increased overall in all adenomyosis patients compared to controls, but this increase was larger and statistically significant only in the adenomyotic group that did not receive long down regulation therapy before embryo transfer. This finding suggests that ultra-long down regulation may reduce the rates of post-implantation pregnancy loss. While previous studies have concluded that prolonged GnRH agonist down regulation is effective in boosting implantation (Tremellen and Russell, 2011; Niu et al., 2013; Park et al., 2016), this study extends these claims by suggesting that this treatment may also improve post-implantation pregnancy health. This observation makes physiological sense given that GnRH receptors are present within adenomyotic tissue, and the use of GnRH agonists and their associate hypo-estrogenic state do induce apoptosis and reduce inflammation within adenomyotic tissue which would otherwise be in direct contact with invading trophoblast (Khan et al., 2010). Furthermore, our routine use of immune-suppressive prednisolone therapy in conjunction with GnRH long down regulation therapy may also assist in reducing hostile immune responses to the invading trophoblast, although we recognize the merits of prednisolone therapy are contentious and currently under considerable debate (Robertson et al., 2016). Finally, as the early pregnancy progesterone levels in the adenomyosis patients were within the accepted healthy pregnancy range, we believe that it is unlikely that additional progesterone luteal support would be an effective treatment for minimizing miscarriage in the setting of adenomyosis. Similar findings have been reported in the setting of recurrent miscarriage, where progesterone support was also ineffective in preventing miscarriage in patients unselected for adenomyosis status (Coomarasamy et al., 2015).
A potential weakness of our study, common to all retrospective studies, is the potential for bias and possible misclassification and error due to inadequate clinical notes. However, our outcome of interest (miscarriage) is not subjective and the determination of adenomyosis status was made prior to the knowledge of pregnancy outcome status, thereby strengthening the veracity of our findings. However, we acknowledge the relatively small sample size of subjects with adenomyosis, especially that sub-set of adenomyosis patients that declined ultra-long down regulation therapy. However, despite this small sample size, the extremely poor pregnancy outcomes in this untreated sub-group (82.4% miscarriage rate) was sufficient to demonstrate a significant difference compared with controls and those adenomyotic patients undergoing long down regulation treatment. Furthermore, due to limited numbers, we were also unable to study whether pregnancy outcomes were influenced by the area of the adenomyotic involvement (focal vs diffuse disease). Future studies are therefore needed to determine if the extent of disease (area of junctional zone involved or thickness of the junctional zone on MRI) also modifies miscarriage risk. These studies would also benefit from having a fixed treatment type to compare the effect of long-down regulation to no down regulation with the same type of endometrial preparation and luteal support in each group. Due to the retrospective nature of our study, this was not possible and therefore we accounted for these factors in our statistical analysis. Importantly, a recent systematic review has not demonstrated any difference in miscarriage rates based on differing FET endometrial preparation regimes (Ghobara et al., 2017). As such, it is unlikely that differences in endometrial preparation regime used were a factor accounting for significant miscarriage rate differences demonstrated in our study. Finally, as the majority of our patients did not undergo laparoscopy prior to IVF treatment, co-existent endometriosis could not be excluded as a potential cause of miscarriage. However, a recent analysis of US registry data has reported no increase in IVF miscarriage rates in women with pure endometriosis-related infertility compared to non-endometriosis causes (Senapati et al., 2016).
Together with previous case-series and cohort studies that have suggested the potential for ultra-long down regulation therapy to augment initial implantation of embryos in IVF treatment (Tremellen and Russell, 2011; Niu et al., 2013; Park et al., 2016), our current findings suggest for the first time that this treatment strategy may reduce early miscarriage rates and thereby augment live-birth rates in patients undergoing IVF.
Conclusion
By using an exclusive PGS euploid embryo transfer experimental approach, and structural equation modelling to assess both direct and indirect effects, we have been able to demonstrate for the first time that adenomyosis is associated with an increased risk of miscarriage, independently of maternal age and BMI. In addition, our study also suggests that ultra-long down regulation inactivation of adenomyosis may be an effective treatment approach to reduce the risk of miscarriage in the setting of adenomyosis. However, prospective randomized controlled trials of GnRH agonist down regulation therapy prior to embryo transfer will be required to provide conclusive evidence of the benefit of this approach.
Supplementary Material
Supplementary data
Supplementary data are available at Human Reproduction Open online.
Authors’ roles
V.S. and K.T. designed the study and performed ethics application; V.S. and K.T. undertook data collection and analysis. R.W. performed statistical mediation analysis and regression modelling. All authors were involved in the writing and editing of the article.
Funding
This study was financially supported by Flinders University Medical School.
Conflict of interest
K.T. is a practicing IVF gynaecologist and holds a minority stake in the publicly listed company Monash IVF. The other authors declare that they have no conflict of interest.
References
- Boots CE, Bernardi LA, Stephenson MD. Frequency of euploid miscarriage is increased in obese women with recurrent early pregnancy loss. Fertil Steril 2014;102:455–459. [DOI] [PubMed] [Google Scholar]
- Brosens JJ, Barker FG, de Souza NM. Myometrial zonal differentiation and uterine junctional zone hyperplasia in the non-pregnant uterus. Hum Reprod Update 1998;4:496–502. [DOI] [PubMed] [Google Scholar]
- Bulletti C, De Ziegler D, Rossi S, Polli V, Massoneau M, Rossi E et al. Abnormal uterine contractility in non-pregnant women. Ann NY Acad Sci 1997;26:223–229. [DOI] [PubMed] [Google Scholar]
- Coomarasamy A, Williams H, Truchanowicz E, Seed PT, Small R, Quenby S et al. A randomized trial of progesterone in women with recurrent miscarriages. N Engl J Med 2015;373:2141–2148. [DOI] [PubMed] [Google Scholar]
- Gardner DK, Lane M, Stevens J, Schlenker T, Schoolcraft WB. Blastocyst score affects implantation and pregnancy outcome: towards a single blastocyst transfer. Fertil Steril 2000;73:1155–1158. [DOI] [PubMed] [Google Scholar]
- Ghobara T, Gelbaya TA, Ayeleke RO. Cycle regimens for frozen-thawed embryo transfer. Cochrane Database Syst Rev 2017;5:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunnala V, Melnick A, Irani M, Reichman D, Schattman G, Davis O, Rosenwaks Z. Sliding scale hCG trigger yields equivalent pregnancy outcomes and reduces ovarian hyperstimulation syndrome: analysis of 10,427 IVF-ICSI cycles. PLoS One 2017;12:e0176019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutierrez-Mateo C, Colls P, Sanchez-Garcia J, Escudero T, Prates R, Ketterson K et al. Validation of microarray comparative genomic hybridization for comprehensive chromosome analysis of embryos. Fertil Steril 2011;95:953–958. [DOI] [PubMed] [Google Scholar]
- Khan KN, Kitajima M, Hiraki K, Fujishita A, Nakashima M, Ishimaru T, Masuzaki H. Cell proliferation effect of GnRH agonist on pathological lesions of women with endometriosis, adenomyosis and uterine myoma. Hum Reprod 2010;25:2878–2890. [DOI] [PubMed] [Google Scholar]
- Kroon B, Harrison K, Martin N, Wong B, Yazdani A. Miscarriage karyotype and its relationship with maternal body mass index, age, and mode of conception. Fertil Steril 2011;95:1827–1829. [DOI] [PubMed] [Google Scholar]
- Kuijsters NPM, Methorst WG, Kortenhorst MSQ, Rabotti C, Mischi M, Schoot BC. Uterine peristalsis and fertility: current knowledge and future perspectives: a review and meta-analysis. Reprod Biomed Online 2017;35:50–71. [DOI] [PubMed] [Google Scholar]
- Leyendecker G, Bilgicyildirim A, Inacker M, Stalf T, Huppert P, Mall G et al. Adenomyosis and endometriosis. Re-visiting their association and further insights into the mechanisms of auto-traumatisation. An MRI study. Arch Gynecol Obstet 2015;291:917–932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez-Conejero JA, Morgan M, Montesinos M, Fortuno S, Meseguer M, Simon C et al. Adenomyosis does not affect implantation, but is associated with miscarriage in patients undergoing oocyte donation. Fertil Steril 2011;96:943–950. [DOI] [PubMed] [Google Scholar]
- Mehasseb MK, Panchal R, Taylor AH, Brown L, Bell SC, Habiba M. Estrogen and progesterone receptor isoform distribution through the menstrual cycle in uteri with and without adenomyosis. Fertil Steril 2011;95:2228–2235. [DOI] [PubMed] [Google Scholar]
- Naftalin J, Hoo W, Pateman K, Mavrelos D, Holland T, Jurkovic D. How common is adenomyosis? A prospective study of prevalence using transvaginal ultrasound in a gynaecology clinic. Hum Reprod 2012;27:3432–3439. [DOI] [PubMed] [Google Scholar]
- Nelson LR, Bulun SE. Estrogen production and action. J Am Acad Dermatol 2001;45:S116–S124. [DOI] [PubMed] [Google Scholar]
- Niu Z, Chen Q, Sun Y, Feng Y. Long-term pituitary downregulation before frozenembryo transfer could improve pregnancy outcomes in women with adenomyosis. Gynecol Endocrinol 2013;29:1026–1030. [DOI] [PubMed] [Google Scholar]
- Norman RJ, Noakes M, Wu R, Davies MJ, Moran L, Wang JX. Improving reproductive performance in overweight/obese women with effective weight management. Hum Reprod Update 2004;10:267–280. [DOI] [PubMed] [Google Scholar]
- Ota H, Igarashi S, Hatazawa J, Tanaka T. Is adenomyosis an immune disease? Hum Reprod Update 1998;4:360–367. [DOI] [PubMed] [Google Scholar]
- Park CW, Choi MH, Yang KM, Song IO. Pregnancy rate in women with adenomyosis undergoing fresh or frozen embryo transfer cycles following gonadotropin-releasing hormone agonist treatment. Clin Exp Reprod Med 2016;43:169–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson SA, Jin M, Yu D, Moldenhauer LM, Davies MJ, Hull ML, Normal RJ. Corticosteroid therapy in assisted reproduction-immune suppression is a faulty premise. Hum Reprod 2016;31:2164–2173. [DOI] [PubMed] [Google Scholar]
- Sakhel K, Abuhamad A. Sonography of adenomyosis. Am Inst Ultrasound Med 2012;31:805–808. [DOI] [PubMed] [Google Scholar]
- Senapati S, Sammel MD, Morse C, Barnhart KT. Impact of endometriosis on in vitro fertilization outcomes: an evaluation of the Society for Assisted Reproductive Technologies Database. Fertil Steril 2016;1:164–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah D, Nagarajan N. Luteal insufficiency in first trimester. Indian J Endocrinol Metab 2013;17:44–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taran FA, Stewart EA, Brucker S. Adenomyosis: epidemiology, risk factors, clinical phenotype and surgical and interventional alternatives to hysterectomy. Geburtshilfe Frauenheilkd 2013;73:924–931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trabert B, Weiss NS, Rudra CB, Scholes D, Holt VL. A case-control investigation of adenomyosis: impact of control group selection on risk factor strength. Women’s Health Issues 2011;21:160–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tremellen K, Pearce K, Zander-Fox D. Increased miscarriage of euploid pregnancies in obese women undergoing cryopreserved embryo transfer. Reprod Biomed Online 2017;31:90–97. [DOI] [PubMed] [Google Scholar]
- Tremellen K, Russell P. Adenomyosis is a potential cause of recurrent implantation failure during IVF treatment. Aust N Z J Obstet Gynaecol 2011;51:280–283. [DOI] [PubMed] [Google Scholar]
- Tremellen KP, Russell P. The distribution of immune cells and macrophages in the endometrium of women with recurrent reproductive failure. II: adenomyosis and macrophages. J Reprod Immunol 2012;93:58–63. [DOI] [PubMed] [Google Scholar]
- Younes G, Tulandi T. Effects of adenomyosis on in vitro fertilization treatment outcomes: a meta-analysis. Fertil Steril 2017;108:483–490. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.