Abstract
Context
Adequate luteal phase progesterone exposure is necessary to induce endometrial changes required for a successful pregnancy outcome. The relationship between low midluteal progesterone concentration and the outcome of live birth in ovarian stimulation with intrauterine insemination (OS-IUI) treatments is not defined.
Objective
To determine the level of midluteal progesterone portending a low chance of live birth after OS-IUI in couples with unexplained infertility.
Design and Setting
Secondary analyses of data from a prospective, randomized, multicenter clinical trial that determined pregnancy outcomes following OS-IUI with clomiphene citrate, letrozole, or gonadotropins for couples with unexplained infertility.
Participants
Couples (n = 900) underwent 2376 OS-IUI cycles during the Assessment of Multiple Intrauterine Gestations from Ovarian Stimulation clinical trial.
Main Outcome Measures
Live birth as it relates to midluteal progesterone level and thresholds below which no live births occur by treatment group.
Results
Thresholds for non-live birth cycles were similar for clomiphene (14.4 ng/mL) and letrozole (13.1 ng/mL) yet were lower for gonadotropin (4.3 ng/mL) treatments. A midluteal progesterone level >10th percentile specific for each treatment group independently was associated with greater odds for a live birth in all OS-IUI cycles (adjusted OR: 2.17; 95% CI: 1.05, 4.48).
Conclusions
During OS-IUI, a low midluteal progesterone level was associated with a low probability of live birth. Thresholds differed by medication, with the lowest threshold for gonadotropin. Several pathophysiologic mechanisms may account for low progesterone levels. Refinement of the predictive range associated with particular ovarian stimulation medications during treatment of unexplained infertility may improve accuracy.
Low midluteal progesterone level is associated with a decreased probability of live birth in women with unexplained infertility undergoing ovarian stimulation‒intrauterine insemination.
Ovarian stimulation (OS) with medications such as clomiphene citrate, letrozole, or gonadotropins coupled with intrauterine insemination (IUI) is often used to assist couples with unexplained infertility who desire to conceive (1, 2). Several factors are associated with pregnancy and live birth after ovarian stimulation with intrauterine insemination (OS-IUI) treatments, including the number of ovulatory-sized follicles that develop in response to ovarian stimulation (3, 4), the age of the female partner (5, 6), and the duration of infertility (7). Adequate luteal phase production of progesterone is required for successful treatment outcomes to induce a receptive secretory phase endometrium necessary for implantation (8).
After OS with gonadotropins (9–11) and with clomiphene citrate (12, 13), abnormal luteal phases as assessed by low serum progesterone levels, short luteal phase length, or both have been reported. In addition, previous investigations have shown that luteal phase supplementation with progesterone may improve pregnancy and live birth rates in OS-IUI treatments (14, 15). These observations suggest that OS-IUI treatment may result in an abnormal luteal phase in some cycles, which may be reflected as lower conception, clinical pregnancy, and live birth outcomes.
The relationship between luteal phase hormone dynamics and pregnancy outcomes in OS-IUI cycles is not clear. In part, this might be because the concept of “luteal insufficiency,” its frequency of occurrence, and its clinical significance are widely debated. There is no universally accepted definition of luteal phase insufficiency (16). Costello et al. (17) could not identify a relationship between midluteal progesterone concentration and the clinical pregnancy rate in 188 OS-IUI treatments with gonadotropins, but they did suggest that a low level may predict treatment failure. Yildirim et al. (18) found a positive association between luteal progesterone level and clinical pregnancy rates in 923 gonadotropin cycles in women with mixed diagnoses, as did Arce et al. (11) in a group of 335 anovulatory women.
To determine the level and frequency of low luteal phase serum progesterone and its relationship to pregnancy outcomes in unexplained infertility, we evaluated midluteal progesterone levels and live birth outcomes for women undergoing OS-IUI as part of the Assessment of Multiple Intrauterine Gestations from Ovarian Stimulation (AMIGOS) clinical trial (19). We hypothesized that the live birth rate would be lower in cycles with a low midluteal progesterone level even after adjustment for factors known to affect success.
Materials and Methods
Patients
This was a secondary analysis of 2376 cycles of 900 participants in the AMIGOS clinical trial (19). The trial design, analysis plan, and baseline characteristics of the participating couples as well as the trial outcomes have been published (19, 20). Briefly, the AMIGOS trial was a prospective, multicenter, randomized clinical trial that evaluated conception, clinical pregnancy, live birth, and multiple gestation rates associated with OS-IUI in couples with unexplained infertility. The trial was conducted at 12 clinical locations in the United States (Clinicaltrials.gov no. NCT01044862). Treatment arms included clomiphene citrate (300 couples), letrozole (299 couples), and gonadotropin (Menopur®, Ferring Pharmaceuticals, Parsippany, NJ) (301 couples). Clomiphene (Teva Pharmaceuticals, North Wales, PA) and letrozole (Novartis Pharmaceuticals, Basal, Switzerland) were acquired and coated by a third-party company (Almac Pharmaceuticals, Craigavon, UK). None of these companies had any involvement in the conduct of the trial. Couples underwent OS-IUI treatment in the randomized assigned study arm until up to four cycles were completed or pregnancy occurred. Participating women were ≥18 to ≤40 years with regular menses, had a normal uterine cavity with at least one patent fallopian tube, and had a male partner with a semen specimen with at least 5 million motile sperm in the ejaculate. Institutional review board approval was obtained at each study site, and all participants provided written informed consent. A data and safety monitoring board monitored the investigation.
Ovarian stimulation protocol and management
Ovarian stimulation consisted of one of the following: (1) clomiphene citrate 100 mg/d starting from day 3 ± 2 of the menstrual cycle for 5 days, (2) letrozole 5 mg/d starting from day 3 ± 2 of the menstrual cycle for 5 days, or (3) gonadotropins 150 U/d starting from day 3 ± 2 of the menstrual cycle of variable duration and dose depending on response. Investigators were given flexibility as to the start date of medications, which was usually influenced by patient availability for baseline and follow-up ultrasonography examinations. In 84% of all cycles, medication was begun on cycle days 2, 3, and 4. Doses of each medication could be adjusted by the treating physician in subsequent cycles. In general, an increase in medication dose in a subsequent cycle occurred when a single follicle had developed in the prior cycle, with the goal of achieving a multifollicular response. A decrease in dose occurred when a prior cycle had been canceled because of an unacceptable risk for multiple gestation pregnancy as defined later.
The criteria for human chorionic gonadotropin (hCG) (Ferring Pharmaceuticals, Parsippany, NJ) administration in all OS-IUI cycles were as follows: (1) the first occurrence of a lead follicle reaching 20 mm in average diameter (the mean of two perpendicular diameters), (2) the first occurrence of two lead follicles >18 mm in average diameter, or (3) the day after the lead follicle reached 18 mm in average diameter. All subjects included in this analysis received a 10,000-IU dose of hCG. Criteria for cycle cancelation (withholding of hCG) were (1) a lead follicle that did not reach a mean diameter of 18 mm after 18 days of treatment (i.e., by cycle day 21), (2) the presence of an endogenous LH surge, (3) an increased risk for ovarian hyperstimulation syndrome or high-order multiple gestation pregnancy based on a serum estradiol level exceeding 3000 pg/mL, or (4) the presence of more than four follicles with a mean diameter >18 mm. IUI was performed within 44 hours after administration of hCG. Serum samples for progesterone assays were obtained 1 week (±1 day) after the IUI. This timing corresponded to 8.7 ± 0.8 days after the hCG injection (day 7.2 ± 0.8 of the luteal phase). Clinical pregnancy was defined as an intrauterine pregnancy with fetal heart motion detected by transvaginal ultrasonography. Live birth was defined as the delivery of a viable infant at ≥20 weeks’ gestation.
Endocrine assays
Blood analytes were determined blinded to the treatment arm and the outcome. Assays for Anti-Müllerian hormone (AMH) were performed on fasting samples obtained on cycle day 3 ± 2 in a pretreatment cycle. Batched samples were analyzed at the Ligand Assay & Analysis Core Laboratory at the University of Virginia with the AMH Gen II ELISA (Beckman Coulter) (19). Intra-assay and interassay coefficients of variation were 3% and 7%, respectively. Progesterone levels (radioimmunoassay; Siemens Corporation) were determined in batch at the University of Virginia. Intra-assay and interassay coefficients of variation were 4% and 7%, respectively.
Statistical analyses
Clinical data from the AMIGOS trial were obtained from the Data Coordinating Center (DCC) of the Collaborative Center for Statistics in Science at Yale University in New Haven, Connecticut. The DCC was responsible for data management in AMIGOS (including data entered at each participating center). Cycles available for analysis were 2695 (886 first cycles, 709 second cycles, 578 third cycles, 467 fourth cycles, and 55 fifth cycles). Fifth cycles occurred in some subjects who had a prior cycle canceled. Total evaluable cycles for our analysis were 2376. Exclusions were as follows: 123 due to hCG injection not given in the cycle of attempt, 19 with a follicular phase length recorded as >21 days, 6 with exogenous progesterone given in the luteal phase, and 171 duplications, entry errors, or missing data.
Defining progesterone thresholds
Efficiency curves were generated to explore the relationship between midluteal progesterone level and outcomes to identify a level below which live births did not occur and ranges for modeling. We graphed the live birth rate for all treatment groups combined and for each treatment group separately for each ng/mL increment in midluteal progesterone concentration. The generated curves show the probability of a live birth on the y-axis at or below the integer progesterone value on the x-axis. This process results in the exclusion of cycles above the given value (both live birth and non-live birth cycles). Thus, with each incremental decrease in progesterone, the total number of cycles decreases because of loss of both positive and negative outcomes. With this approach, it is possible for live birth rates to go up and down as progressively lower thresholds are evaluated. Because the efficiency curves suggested decreased live birth rates at approximately the 10th percentile for each treatment group independently, these 10th percentile thresholds (15.8 ng/mL for clomiphene cycles, 14.8 ng/mL for letrozole cycles, and 9.1 ng/mL for gonadotropin cycles) were used for subsequent modeling. These same thresholds are consistent with those in prior literature (21–24).
Data are frequency and percentages for categorical variables for each group comparison. χ2 analysis or Fisher exact test compared differences between groups. Means ± SD are reported for continuous variables. Wilcoxon rank sum test was used to compare differences between two groups, and ANOVA or the Kruskal-Wallis test was used to evaluate differences among groups of three or more.
Modeling
Regression modeling was used to evaluate the relationship between midluteal progesterone level and the outcome of live birth in all cycles combined (2376 cycles). Then, because clomiphene citrate and letrozole (CC+L) treatment cycles had similar relationships between midluteal progesterone level and live birth, these cycles were modeled together (1671 cycles), whereas gonadotropin cycles were modeled separately (705 cycles).
Generalized estimating equation (GEE) modeling evaluated repetitive cycles in the same couples longitudinally to determine the association of midluteal phase progesterone values with subsequent live birth after adjustment for covariates. GEE modeling does not assume independence of cycles and adjusts for repetitive cycles in the same couple. The number of cycles for individuals who did not conceive after one attempt ranged from one to four. In each treatment group, models were developed that (1) adjusted for the number of ovulatory-sized follicles (16 mm or greater), (2) adjusted for estradiol level the day of the hCG trigger, and (3) adjusted for variables that differed between “low” (≤10th percentile) and “normal” progesterone cycles as defined previously. These covariates included age, baseline body mass index (BMI), number of follicles 16 mm or greater at the time of the hCG trigger in the cycle of analysis, months attempting to conceive before entry into the study, treatment cycle number in the study, treatment medication, and length of the follicular phase in the current cycle before hCG injection. These characteristics were compared by progesterone group for each treatment. The day of progesterone measurement differed in gonadotropin cycles by categorization of the cycle as having low or normal progesterone; therefore, this too was included in the GEE modeling. Analyses were performed utilizing the Statistical Analysis System, version 9.4 (SAS Institute, Cary, NC) and NCSS 10 Statistical Software (2015) (NCSS, LLC, Kaysville, Utah), with a P value < 0.05 indicating statistical significance.
Results
Characteristics of all cycles by treatment group
Baseline clinical and treatment cycle characteristics by treatment group are displayed in Table 1. BMI was statistically significantly different across the treatment groups, but other baseline characteristics, including age, AMH, and duration of infertility, were comparable (Table 1). Treatment cycle-specific characteristics that differed across the treatment groups include the number of follicles ≥16 mm that developed during the stimulation, follicular phase length, estradiol level the day of the hCG trigger, and midluteal progesterone concentration (Table 1). Live birth rate per cycle differed by treatment group (7.4%, 6.5%, and 12.1% per cycle in the clomiphene citrate, letrozole, and gonadotropin cycles, respectively) (Table 1).
Table 1.
Characteristics of Cycles by Treatment Group
| Clomiphene Cycles (n = 828) | Letrozole Cycles (n = 843) | Gonadotropin Cycles (n = 705) | P Value | |
|---|---|---|---|---|
| Age | 32.1 (4.3) | 32.2 (4.2) | 32.4 (4.0) | 0.461 |
| BMI, kg/m2 | 27.2 (7.2) | 27.6 (6.7) | 26.7 (6.5) | 0.004 |
| No. of follicles ≥16 mm | 2.0 (1.0) | 1.7 (0.8) | 2.2 (1.4) | <0.001 |
| Follicular phase length, d | 12.2 (2.0) | 11.9 (2.0) | 12.0 (2.3) | 0.048 |
| AMH, ng/mL | 2.7 (2.2) | 2.6 (1.9) | 2.4 (1.9) | 0.170 |
| Duration of infertility, mo | 34.7 (24.3) | 34.7 (25.6) | 35.7 (26.4) | 0.566 |
| Peak estradiol, pg/mL | 625.9 (379.1) | 162.3 (111.4) | 731.8 (503.6) | <0.001 |
| Peak estradiol, pg/mL/follicle ≥16 mm | 341.1 (217.7) | 110.8 (87.4) | 386.7 (272.1) | <0.001 |
| Midluteal progesterone, ng/mL | 40.2 (26.0) | 30.4 (16.1) | 35.2 (32.9) | <0.001 |
| Day of progesteronea | 8.7 (0.8) | 8.8 (0.8) | 8.7 (0.8) | 0.021 |
| Achieved pregnancy, n (%) | 93 (11.2) | 77 (9.1) | 121 (17.2) | <0.001 |
| Clinical pregnancy, n (%) | 61 (7.4) | 56 (6.6) | 86 (12.2) | <0.001 |
| Live birth, n (%) | 61 (7.4) | 55 (6.5) | 85 (12.1) | <0.001 |
Comparisons between groups are by parametric or nonparametric tests as appropriate. Age, BMI, AMH, and duration of infertility are baseline characteristics. Other variables are cycle specific. Values are expressed as mean (SD) unless otherwise indicated.
Day of progesterone is the interval between the day of progesterone blood draw and the hCG injection.
Efficiency curves for live births by treatment group and midluteal progesterone level (unadjusted analyses)
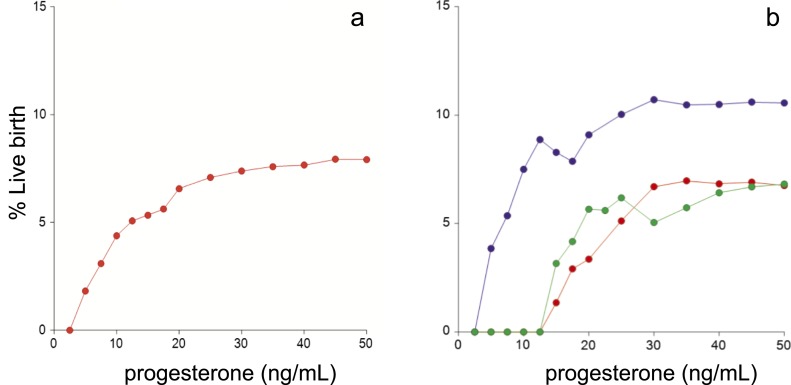
Efficiency curves for live births for all cycles combined and by individual treatment groups (Fig. 1) demonstrated an increase in the live birth rate in all treatment groups with higher midluteal progesterone levels. There were no live births in the clomiphene citrate cycles when the midluteal progesterone level was <14.4 ng/mL, in the letrozole cycles when it was <13.1 ng/mL, and in the gonadotropin cycles when it was <4.3 ng/mL.
Figure 1.
Efficiency curves for live births by midluteal progesterone level. Efficiency curves were calculated (a) for all treatment groups combined and (b) for each treatment group individually (blue represents gonadotropin cycles, green represents letrozole cycles, and red represents clomiphene citrate cycles). The generated curves show the probability of a live birth on the y-axis at or below the integer progesterone value on the x-axis. This process results in the exclusion of cycles above the given value (both live birth and non‒live birth cycles). Thus, with each incremental decrease in progesterone, the total number of cycles decreases because of loss of both positive and negative outcomes. With this approach, it is possible for live birth rates to go up and down as progressively lower thresholds are evaluated.
We used the lowest 10th percentile of midluteal progesterone concentration for each unique treatment group to define low vs normal midluteal progesterone cycles. These thresholds were consistent with the developed efficiency curves and were 15.8, 14.8, and 9.1 ng/mL for clomiphene citrate, letrozole, and gonadotropin cycles, respectively. Using these cutpoints, live birth rates in low vs normal midluteal progesterone cycles were 1.2% vs 8.1% (P = 0.024) in the clomiphene cycles, 2.4% vs 7.0% (P = 0.158) in the letrozole cycles, and 8.5% vs 12.5% (P = 0.325) in the gonadotropin cycles (Supplemental Table 1).
Characteristics of cycles by progesterone category
Characteristics of cycles stratified by treatment groups and by midluteal progesterone category are presented in Table 2. Comparisons were performed for all treatment cycles combined (n = 2376). We also performed comparisons (and subsequent modeling) in the CC+L cycles combined (n = 1671), given the similar relationship between midluteal progesterone and live birth in these treatment cycles as demonstrated by efficiency curves (Fig. 1). Because gonadotropin cycles had higher live birth rates at lower progesterone concentrations, these cycles (n = 705) were modeled independently.
Table 2.
Characteristics of Cycles by Progesterone Category and Ovarian Stimulation Medication
| Clomiphene, Letrozole, and Gonadotropin Cycles (n = 2376) | P Value | Clomiphene and Letrozole Cycles (n = 1671) | P Value | Gonadotropin Cycles (n = 705) | P Value | ||||
|---|---|---|---|---|---|---|---|---|---|
| Progesterone group, n cyclesa | Low | Normal | Low | Normal | Low | Normal | |||
| 238 | 2138 | 167 | 1504 | 71 | 634 | ||||
| Age | 32.7 (4.3) | 32.2 (4.2) | 0.031 | 32.9 (4.4) | 32.1 (4.2) | 0.019 | 32.4 (4.1) | 32.4 (4.0) | 0.716 |
| BMI | 31.4 (8.4) | 26.7 (6.5) | <0.001 | 32.0 (8.9) | 26.9 (6.5) | <0.001 | 30.0 (7.1) | 26.3 (6.3) | <0.001 |
| No. of follicles ≥16 mm | 1.6 (0.8) | 2.0 (1.1) | <0.001 | 1.5 (0.7) | 1.9 (0.9) | <0.001 | 1.9 (1.1) | 2.2 (1.5) | 0.058 |
| Follicular phase length | 12.8 (2.6) | 12.0 (2.0) | <0.001 | 12.9 (2.5) | 12.0 (1.9) | <0.001 | 12.6 (2.7) | 11.9 (2.3) | 0.005 |
| AMH, ng/mL | 2.3 (1.7) | 2.6 (2.0) | 0.115 | 2.3 (1.8) | 2.6 (2.1) | 0.071 | 2.3 (1.5) | 2.5 (2.0) | 0.957 |
| Duration of infertility, mo | 37.9 (25.0) | 34.7 (25.4) | 0.022 | 36.8 (24.6) | 34.5 (25.0) | 0.193 | 40.3 (26.0) | 35.2 (26.4) | 0.028 |
| Peak estradiol, pg/mL | 357.4 (353.4) | 507.6 (443.7) | <0.001 | 258.4 (239.0) | 405.8 (370.1) | <0.001 | 588.9 (457.0) | 747.9 (506.4) | 0.002 |
| Peak estradiol/follicle ≥16 mm | 229.6 (178.3) | 277.8 (241.6) | 0.064 | 189.1 (143.3) | 229.1 (206.6) | 0.088 | 326.4 (214.1) | 393.3 (277.0) | 0.059 |
| Medication dose | |||||||||
| Clomiphene, mg | 572.3 (132.6) | 532.9 (110.4) | 0.001 | 572.3 (132.6) | 532.9 (110.4) | 0.001 | NA | NA | NA |
| Letrozole, mg | 28.6 (6.0) | 28.3 (6.0) | 0.439 | 28.6 (6.0) | 28.3 (6.0) | 0.439 | NA | NA | NA |
| Gonadotropins, IU | 788.0 (642.0) | 635.2 (385.1) | 0.049 | NA | NA | NA | 788.0 (642.0) | 635.2 (385.1) | 0.049 |
| Day of progesteroneb | 8.6 (0.9) | 8.7 (0.8) | 0.117 | 8.8 (0.9) | 8.7 (0.8) | 0.728 | 8.3 (0.8) | 8.7 (0.8) | 0.001 |
| Achieved pregnancy, n (%) | 12 (5.0) | 279 (13.1) | <0.001 | 6 (3.6) | 164 (10.9) | 0.003 | 6 (8.5) | 115 (18.1) | 0.040 |
| Clinical pregnancy, n (%) | 9 (3.8) | 194 (9.1) | 0.006 | 3 (1.8) | 114 (7.6) | 0.005 | 6 (8.5) | 80 (12.6) | 0.309 |
| Live birth, n (%) | 9 (3.8) | 192 (9.0) | 0.006 | 3 (1.8) | 113 (7.5) | 0.003 | 6 (8.5) | 79 (12.5) | 0.325 |
Values are expressed as means (SD) unless otherwise indicated. Comparisons between groups are by parametric or nonparametric tests as appropriate. Age, BMI, AMH, and duration of infertility are baseline characteristics. Other variables are cycle specific.
Abbreviation: NA, not applicable.
Cutpoints to define “low” vs “normal” midluteal progesterone cycles were the 10th percentile; cutpoints were consistent with the efficiency curves for each treatment group independently. Cutpoints were 15.8, 14.8, and 9.1 ng/mL for clomiphene, letrozole, and gonadotropin cycles, respectively.
Day of progesterone is the interval between the day of the progesterone blood draw and the hCG injection.
For all cycles, age, BMI, number of follicles ≥16 mm on the day of the hCG trigger, follicular phase length, duration of infertility, and peak estradiol level differed by progesterone categories (Table 2). For the CC+L cycles, when the low and normal midluteal progesterone groups were compared, differences in each of the baseline and cycle-specific variables were similar to those found between low and normal progesterone groups in all cycles combined except for duration of infertility, which was not significantly different (Table 2). In the gonadotropin cycles, BMI, duration of infertility, peak estradiol level, and follicular phase length were different (Table 2). Pregnancy loss beyond an initially rising hCG level and clinical pregnancy loss rates were comparable across the progesterone categories for each of the treatment groups (Table 2). In all cycles combined and in the oral medication-only cycles, live birth rates were lower in the low than in the normal progesterone cycles. In the gonadotropin only cycles, the live birth rate was also lower in the low progesterone cycles; however, this difference was not statistically significant (Table 2). When a single follicle developed during ovarian stimulation, live birth rates were lower in the low progesterone than in the normal progesterone cycles. However, these differences in the live birth rates were not statistically significant: in all cycles, 4.1% vs 6.8%; in CC+L cycles, 3.2% vs 6.4%; and in gonadotropin cycles, 6.9% vs 7.5%; low vs normal progesterone, respectively. Using the respective cutpoints for all treatment cycles, a single midluteal progesterone level had a sensitivity of 90.5%, a specificity of 16.4%, a positive predictive value of 9.1%, and a negative predictive value of 94.9%.
Of interest, 18 women had repetitive low progesterone value cycles. These occurred in four treatment cycles in one woman, in three treatment cycles in five women, and in two cycles of OS-IUI in 12 women. However, the majority of the low progesterone cycles (195 cycles, 82%) were not repetitive.
Multivariable analyses
The GEE analysis results are shown in Table 3. For all treatment cycles, with low progesterone as the referent group, a normal midluteal progesterone level was associated with greater odds of live birth (OR: 2.47; 95% CI: 1.28, 4.79). This was true even after adjustment for the number of follicles ≥16 mm on the day of hCG trigger alone (adjusted model 1) or after adjustment for peak estradiol level (an alternative means of assessing the robustness of stimulation; adjusted model 2). Increased odds of live birth was also noted in the normal vs low progesterone cycles after adjustment for the variables noted to significantly differ between progesterone groups (i.e., age, BMI, the number of follicles ≥16 mm on the day of the hCG trigger, follicular phase length, duration of infertility, treatment medication, and treatment cycle number; adjusted OR (AOR): 2.17; 95% CI: 1.05, 4.48; adjusted model 3). Replacing the number of follicles ≥16 mm on the day of the hCG trigger with peak estradiol level in the model gave similar inference (AOR: 2.13; 95% CI: 1.04, 4.39) (data not shown in table).
Table 3.
ORs and 95% CIs for the Probabilities Between Progesterone Level and Live Birth by Treatment Group
| Progesterone Group a | Low | Normal |
|---|---|---|
| Clomiphene, letrozole, and gonadotropin cycles (n = 2376) | ||
| Unadjusted model | Ref | 2.47 (1.28, 4.79) |
| Adjusted model 1 | Ref | 2.45 (1.22, 4.92) |
| Adjusted model 2 | Ref | 2.46 (1.23, 4.96) |
| Adjusted model 3 | Ref | 2.17 (1.05, 4.48) |
| Clomiphene and letrozole cycles (n = 1671) | ||
| Unadjusted model | Ref | 4.40 (1.42, 13.64) |
| Adjusted model 1 | Ref | 4.07 (1.31, 12.62) |
| Adjusted model 2 | Ref | 4.12 (1.32, 12.89) |
| Adjusted model 3 | Ref | 2.09 (1.01, 4.34) |
| Gonadotropin cycles (n = 705) | ||
| Unadjusted model | Ref | 1.50 (0.66, 3.43) |
| Adjusted model 1 | Ref | 1.58 (0.65, 3.83) |
| Adjusted model 2 | Ref | 1.60 (0.66, 3.85) |
| Adjusted model 3 | Ref | 1.36 (0.51, 3.66) |
GEEs to estimate ORs and 95% CIs. Adjusted model 1 is adjusted for the number of follicles ≥16 mm on the day of the hCG trigger. Adjusted model 2 is adjusted for estradiol level the day of the hCG trigger. Adjusted model 3 is adjusted for variables that significantly differ by progesterone group. For all cycles: the number of follicles ≥16 mm on the day of the hCG trigger, age, duration of infertility, treatment medication, BMI, follicular phase length, and treatment cycle number; for clomiphene and letrozole cycles: the number of follicles ≥16 mm on the day of the hCG trigger, age, duration of infertility, treatment medication, BMI, follicular phase length, and treatment cycle number; and for gonadotropin cycles: the number of follicles ≥16 mm on the day of the hCG trigger, age, duration of infertility, BMI, follicular phase length, luteal phase day of progesterone level, and treatment cycle number. Boldface indicates statistical significance.
Abbreviation: Ref, referent group.
Cutpoints to define “low” vs “normal” midluteal progesterone cycles were the lowest 10th percentile for each medication independently and were consistent with the efficiency curves for each treatment group independently. Cutpoints were ≤15.8, ≤14.8, and ≤9.1 ng/mL for clomiphene, letrozole, and gonadotropin cycles, respectively.
In the oral medications (CC+L) group, cycles with normal midluteal progesterone had approximately twofold to fourfold greater odds of a live birth in the unadjusted and adjusted models (Table 3). When normal to low progesterone groups were compared, the increased odds of a live birth held after adjustment for the number of follicles ≥16 mm on the day of the hCG trigger, age, duration of infertility, treatment medication, BMI, follicular phase length, and treatment cycle number (AOR: 2.09; 95% CI: 1.01, 4.34). As with the analyses of all cycles combined, replacing the number of follicles ≥16 mm on the day of the hCG trigger with peak estradiol level did not change the inference (AOR: 2.07; 95% CI: 1.01, 4.26).
For the gonadotropin treatment group, cycles with a normal midluteal progesterone cycle had ∼1.3- to 1.6-fold greater odds of a live birth compared with the low progesterone cycles in the unadjusted and adjusted models (Table 3); however, this difference was not significant.
Discussion
In a cohort of women with unexplained infertility, we found that a low midluteal progesterone level (≤15.8, ≤14.8, and ≤9.1 ng/mL for the clomiphene citrate, letrozole, and gonadotropin cycles, respectively) in OS-IUI treatment cycles was associated with lower probability of obtaining a live birth. In addition, there were no live births with a midluteal progesterone <14.4, <13.1, and <4.3 ng/mL in the clomiphene citrate, letrozole, and gonadotropin cycles, respectively. To our knowledge, this is the largest investigation of the relationship between a single midluteal progesterone level in OS-IUI treatment cycles and the outcome of live birth and the only one focusing on women with unexplained infertility.
Our finding of lower live birth rate below the determined cutpoints held after adjustments for the number of ovulatory-sized follicles that developed as well as peak serum estradiol level during stimulation. We considered including both the number of ovulatory follicles and peak serum estradiol level in the same model. However, because both are collinear, adjusting for both in the same model would weaken our inference because of overadjustment. The consistency of our findings after adjustment for these alternative measures of the robustness of stimulation suggests that the observed lower live birth rate was not simply due to fewer follicles ovulating in low progesterone cycles. In addition, the live birth rate was lower in low progesterone cycles even when a single follicle developed during stimulation, although these differences did not reach statistical significance. The negative predictive value of a low midluteal progesterone cycle was 94.9%
Prior investigations have suggested similar cutoffs (10 to 15 ng/mL) to differentiate low from normal midluteal progesterone levels (21–23). Of interest, our identified thresholds are within the range of those deemed necessary to induce normal midluteal phase endometrial gene expression in normally cycling reproductive-age women without a history of infertility (range, 9.4 to 18.1 ng/mL) (24). A single midluteal progesterone value is a crude marker of luteal phase progesterone production, yet it was the best indicator available to us in this data set to assess luteal phase function. In natural unstimulated cycles, progesterone levels fluctuate dramatically over a period of hours in the luteal phase because of its pulsatile secretion (25), which makes a single progesterone level obtained in the luteal phase difficult to interpret. These fluctuations in serum progesterone levels may be attenuated in cycles triggered with hCG and/or after treatment with OS medications (26), and that is possibly why the single level here is more reflective of prognosis. Pooled daily values integrated over the luteal phase could have provided a more accurate determination of luteal progesterone production. However, this would have required multiple additional visits, which would have increased study participant burden and was not obtained in the AMIGOS study.
Differences in the performance of the midluteal progesterone thresholds across treatment medications are of interest. In the oral medication group, a low midluteal progesterone value (≤15.8 and 14.8 ng/mL in the clomiphene and letrozole cycles, respectively) was clearly associated with lower chances of a live birth in both the unadjusted and adjusted models. However, in the gonadotropin treatment group, odds for a live birth increased with a progesterone level of >9.1 ng/mL in the models, although it was not statistically significant. Whether this poorer performance is due to the identification of the incorrect threshold or is simply a sample size limitation is uncertain. There are inherent challenges in detecting significant differences in live birth outcomes between progesterone groups with any of the medications because the absolute differences between groups are small. This is particularly true in the gonadotropin group, wherein the difference in live birth outcomes between progesterone groups was ∼4%. CC+L groups were combined for analyses to mitigate potential sample size issues. The overall low live birth rate per cycle with any OS-IUI treatments is reflected in the low positive predictive value of a normal midluteal progesterone level (9.1%).
An unexpected finding of the present investigation is that live births occurred at markedly lower progesterone levels in cycles when ovarian stimulation was conducted with gonadotropins compared with oral medications. Although our study design could not address this specific finding, it is possible that through their antiestrogenic effects (i.e., estrogen receptor antagonism associated with clomiphene and the inhibition of estrogen production with letrozole) the oral medications resulted in an endometrium that was less responsive to progesterone. Thus, higher progesterone levels are necessary to induce appropriate endometrial receptivity. Alternatively, but perhaps less likely, it is possible that embryo quality is superior after ovarian stimulation with gonadotropins compared with the oral medications.
Within a given cycle, midluteal progesterone concentration is a consequence of the number of corpora lutea producing progesterone as well as their function, which may vary from cycle to cycle and by treatment strategy. In the present investigation, differences in both cycle-specific and baseline characteristics were noted between cycles with normal or low progesterone. Specifically, low progesterone cycles were more common in women with a greater BMI. They were also associated with longer follicular phases than in normal progesterone cycles. Although longer follicular phase length and/or BMI was not independently associated with live birth, it is possible that both are mechanistically related to the development of a low midluteal progesterone cycle.
It was previously hypothesized that an abnormal luteal phase could occur during OS treatments because of high estradiol and/or progesterone concentrations during stimulation causing negative feedback on the hypothalamic-pituitary axis. Secretion of LH necessary for continued progesterone production from the corpora lutea during the luteal phase would be inhibited (27). We found no evidence to support this hypothesis, as normal progesterone cycles were associated with higher peak estradiol levels the day of hCG injection.
If a low midluteal progesterone level is associated with a lower live birth rate in OS-IUI treatments, what are the potential mechanisms? It is possible that a low midluteal progesterone level is simply a reflection of fewer ovulatory follicles and/or poor granulosa cell function in a given cycle for any given patient, subsequently leading to a lower chance for a live birth. In the present investigation, we adjusted for stimulation quality by adjusting both the number of ovulatory-sized follicles and peak estradiol concentrations. After either adjustment, the relationship between midluteal progesterone level and live birth outcome was similar. This suggests that the observed association was not simply due to follicular phase events.
It remains unclear whether a midluteal progesterone concentration is a surrogate marker for factors that increase the chance for live birth, or alternatively, whether higher progesterone production is directly responsible for the observed difference in live birth rate in the AMIGOS trial. If the latter hypothesis is true, then luteal progesterone supplementation would be anticipated to improve outcomes in OS-IUI cycles. A recent meta-analysis suggested that there are improved outcomes in gonadotropin-IUI treatments when supplemented with luteal progesterone (28). In cycles with clomiphene citrate or letrozole, this was not shown. However, only a single study has evaluated progesterone supplementation in letrozole cycles, and none of the clomiphene studies have included live birth as the outcome of interest (28, 29).
Our investigation has several strengths and limitations. The AMIGOS clinical trial was a large, prospective, multicenter, randomized, controlled trial with a large number of evaluable cycles. Individual treatment cycles and participant characteristics were well characterized, and the treatments were standardized, including utilization of the same criteria for administration of the same dose of hCG in all the cycles. In addition, all of the progesterone assays were performed by a single laboratory, avoiding concerns regarding variability between sites. Because all cycles in the AMIGOS study were triggered with hCG, we cannot extrapolate our findings to those cycles wherein ovulation occurs as the result of a spontaneous LH surge, nor can they be generalized to women with diagnoses other than unexplained infertility. Because the gonadotropin preparation used in AMIGOS contained LH activity, we cannot extrapolate our findings to preparations consisting of pure FSH activity. Another limitation is the possibility that residual hCG from the ovulatory bolus or alternatively due to an early pregnancy contributed to the midluteal progesterone levels obtained in treatment cycles. However, the average day of the midluteal progesterone measurement was luteal phase day 7.2, making both explanations unlikely (30).
In summary, in this large prospective clinical trial designed to evaluate the effectiveness of and multiple pregnancy rates after OS-IUI treatment in couples with unexplained infertility, we found that a low midluteal progesterone level is an important predictor of reduced odds for a live birth. Additional investigations are necessary to confirm this association and to help refine clinically useful cutoff values for a low midluteal progesterone level by stimulation protocol associated with lack of success in OS-IUI treatment cycles.
Supplementary Material
Acknowledgments
Financial Support: This work was supported by the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) Grants no. U10 HD077680 (to K.R.H.), U10 HD39005 (to M.P.D.), U10 HD38992 (to R. S. L.), U10 HD27049 (to C.C.), U10 HD38998 (to R.A.), U10 HD055942 (to R.D.R.), U10 HD055944 (to P.C.), U10 HD055936 (to G.M.C.), and U10 HD055925 (to H.Z.). This research was made possible by the funding of the American Recovery and Reinvestment Act. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NICHD, National Institutes of Health (NIH), or the United States Department of Defense.
Clinical Trial Information : ClinicalTrials.gov no. NCT01044862 (registered 8 January 2010).
Author Contributions: K.R.H. contributed to the study design, acquisition of data, analysis, manuscript preparation, and final approval of the version. E.E. contributed to the design and analysis of the data, critical revision, and approval of the manuscript. V.B., S.T., R.S.L., C.C., R.A., R.D.R., P.C., G.M.C., and N.S. contributed to the acquisition of data, critical revision, and final approval of the manuscript. M.J.H. contributed to the study concept, critical revision, and final approval of the manuscript. S.C. contributed to the statistical analyses, interpretation and manuscript revision, and final approval of the manuscript. M.P.D. contributed to the acquisition of data, critical revision, and final approval of the manuscript and served as primary investigator for AMIGOS. H.Z. served as DCC statistician and contributed to the interpretation of results and manuscript revision. R.A.W. contributed to the study design, lead statistical analyses, manuscript preparation, and approval of the final version.
Current Affiliations: M.P. Diamond’s current institution is the Department of Obstetrics and Gynecology, Augusta University, Augusta, Georgia 30912. R. Alvero’s current institution is the Department of Obstetrics and Gynecology, Brown University, Providence, Rhode Island 02905. P. Casson’s current institution is Northeastern Reproductive Medicine, Colchester, Vermont 05446. G.M. Christman’s current institution is the Department of Obstetrics and Gynecology, Shands Hospital, University of Florida, Gainesville, Florida 32611.
Disclosure Summary: G.M.C. reports grants from AbbVie Pharmaceuticals and Bayer Pharmaceuticals outside the submitted work. C.C. reports grants from the NIH/NICHD during the conduct of the study and personal fees from ASRM outside the submitted work. M.P.D. reports grants from the NIH/NICHD during the conduct of the study. E.E. reports salary from the NICHD during the conduct of the study. K.R.H. reports grants from the NIH/NICHD during the conduct of the study and grants from Roche Diagnostics and Ferring International Pharmascience Center US outside the submitted work. R.S.L. reports personal fees from Odega, Kindex, Fractyl, and Bayer, grants from Ferring Pharmaceuticals, and personal fees from AbbVie outside the submitted work. R.D.R. reports grants from the NIH during the conduct of the study and grants from AbbVie outside the submitted work. H.Z. reports grants from the NIH during the conduct of the study. The remaining authors have nothing to disclose.
Glossary
Abbreviations:
- AMH
Anti-Müllerian hormone
- AMIGOS
Assessment of Multiple Intrauterine Gestations from Ovarian Stimulation
- AOR
adjusted OR
- BMI
body mass index
- CC+L
clomiphene citrate and letrozole
- DCC
Data Coordinating Center
- GEE
generalized estimating equation
- hCG
human chorionic gonadotropin
- IUI
intrauterine insemination
- OS
ovarian stimulation
- OS-IUI
ovarian stimulation with intrauterine insemination
References
- 1. Guzick DS, Sullivan MW, Adamson GD, Cedars MI, Falk RJ, Peterson EP, Steinkampf MP. Efficacy of treatment for unexplained infertility. Fertil Steril. 1998;70(2):207–213. [DOI] [PubMed] [Google Scholar]
- 2. Berker B, Kahraman K, Taskin S, Sukur YE, Sonmezer M, Atabekoglu CS. Recombinant FSH versus clomiphene citrate for ovarian stimulation in couples with unexplained infertility and male subfertility undergoing intrauterine insemination: a randomized trial. Arch Gynecol Obstet. 2011;284(6):1561–1566. [DOI] [PubMed] [Google Scholar]
- 3. Park SJ, Alvarez JR, Weiss G, Von Hagen S, Smith D, McGovern PG. Ovulatory status and follicular response predict success of clomiphene citrate-intrauterine insemination. Fertil Steril. 2007;87(5):1102–1107. [DOI] [PubMed] [Google Scholar]
- 4. Ghesquiere SL, Castelain EG, Spiessens C, Meuleman CL, D’Hooghe TM. Relationship between follicle number and (multiple) live birth rate after controlled ovarian hyperstimulation and intrauterine insemination. Am J Obstet Gynecol. 2007;197(6):589.e1–589.e5. [DOI] [PubMed] [Google Scholar]
- 5. Brzechffa PR, Daneshmand S, Buyalos RP. Sequential clomiphene citrate and human menopausal gonadotrophin with intrauterine insemination: the effect of patient age on clinical outcome. Hum Reprod. 1998;13(8):2110–2114. [DOI] [PubMed] [Google Scholar]
- 6. Sahakyan M, Harlow BL, Hornstein MD. Influence of age, diagnosis, and cycle number on pregnancy rates with gonadotropin-induced controlled ovarian hyperstimulation and intrauterine insemination. Fertil Steril. 1999;72(3):500–504. [DOI] [PubMed] [Google Scholar]
- 7. Hansen KR, He AL, Styer AK, Wild RA, Butts S, Engmann L, Diamond MP, Legro RS, Coutifaris C, Alvero R, Robinson RD, Casson P, Christman GM, Huang H, Santoro N, Eisenberg E, Zhang H; Eunice Kennedy Shriver National Institute of Child Health and Human Development Reproductive Medicine Network . Predictors of pregnancy and live-birth in couples with unexplained infertility after ovarian stimulation-intrauterine insemination. Fertil Steril. 2016;105(6):1575–1583.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Large MJ, DeMayo FJ. The regulation of embryo implantation and endometrial decidualization by progesterone receptor signaling. Mol Cell Endocrinol. 2012;358(2):155–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Duffy DA, Manzi D, Benadiva C, Maier D, Saunders M, Nulsen J. Impact of leuprolide acetate on luteal phase function in women undergoing controlled ovarian hyperstimulation and intrauterine insemination. Fertil Steril. 2006;85(2):407–411. [DOI] [PubMed] [Google Scholar]
- 10. Olson JL, Rebar RW, Schreiber JR, Vaitukaitis JL. Shortened luteal phase after ovulation induction with human menopausal gonadotropin and human chorionic gonadotropin. Fertil Steril. 1983;39(3):284–291. [PubMed] [Google Scholar]
- 11. Arce JC, Balen A, Platteau P, Pettersson G, Andersen AN. Mid-luteal progesterone concentrations are associated with live birth rates during ovulation induction. Reprod Biomed Online. 2011;22(5):449–456. [DOI] [PubMed] [Google Scholar]
- 12. Cook CL, Schroeder JA, Yussman MA, Sanfilippo JS. Induction of luteal phase defect with clomiphene citrate. Am J Obstet Gynecol. 1984;149(6):613–616. [DOI] [PubMed] [Google Scholar]
- 13. Wu CH, Winkel CA. The effect of therapy initiation day on clomiphene citrate therapy. Fertil Steril. 1989;52(4):564–568. [DOI] [PubMed] [Google Scholar]
- 14. Erdem A, Erdem M, Atmaca S, Guler I. Impact of luteal phase support on pregnancy rates in intrauterine insemination cycles: a prospective randomized study. Fertil Steril. 2009;91(6):2508–2513. [DOI] [PubMed] [Google Scholar]
- 15. Maher MA. Luteal phase support may improve pregnancy outcomes during intrauterine insemination cycles. Eur J Obstet Gynecol Reprod Biol. 2011;157(1):57–62. [DOI] [PubMed] [Google Scholar]
- 16. Practice Committee of the American Society for Reproductive Medicine The clinical relevance of luteal phase deficiency: a committee opinion. Fertil Steril. 2012;98(5):1112–1117. [DOI] [PubMed] [Google Scholar]
- 17. Costello MF, Emerson S, Lukic J, Sjoblom P, Garrett D, Hughes G, Steigrad S. Predictive value of mid luteal progesterone concentration before luteal support in controlled ovarian hyperstimulation with intrauterine insemination. Aust N Z J Obstet Gynaecol. 2004;44(1):51–56. [DOI] [PubMed] [Google Scholar]
- 18. Yildirim G, Turkgeldi LS, Koroglu N, Güler S, Aldikactioglu Talmac M Predictive factors for pregnancy outcome following controlled ovarian stimulation and intrauterine insemination. J Pak Med Assoc. 2017;67(3):422–427. [PubMed] [Google Scholar]
- 19. Diamond MP, Legro RS, Coutifaris C, Alvero R, Robinson RD, Casson P, Christman GM, Ager J, Huang H, Hansen KR, Baker V, Usadi R, Seungdamrong A, Bates GW, Rosen RM, Haisenleder D, Krawetz SA, Barnhart K, Trussell JC, Ohl D, Jin Y, Santoro N, Eisenberg E, Zhang H; NICHD Reproductive Medicine Network . Letrozole, gonadotropin, or clomiphene for unexplained infertility. N Engl J Med. 2015;373(13):1230–1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Diamond MP, Legro RS, Coutifaris C, Alvero R, Robinson RD, Casson P, Christman GM, Ager J, Huang H, Hansen KR, Baker V, Usadi R, Seungdamrong A, Bates GW, Rosen RM, Haisonleder D, Krawetz SA, Barnhart K, Trussell JC, Jin Y, Santoro N, Eisenberg E, Zhang H; National Institute of Child Health and Human Development (NICHD) Reproductive Medicine Network . Assessment of multiple intrauterine gestations from ovarian stimulation (AMIGOS) trial: baseline characteristics. Fertil Steril. 2015;103(4):962–973.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Jordan J, Craig K, Clifton DK, Soules MR. Luteal phase defect: the sensitivity and specificity of diagnostic methods in common clinical use. Fertil Steril. 1994;62(1):54–62. [DOI] [PubMed] [Google Scholar]
- 22. Radwanska E, Hammond J, Smith P. Single midluteal progesterone assay in the management of ovulatory infertility. J Reprod Med. 1981;26(2):85–89. [PubMed] [Google Scholar]
- 23. Hull MG, Savage PE, Bromham DR, Ismail AA, Morris AF. The value of a single serum progesterone measurement in the midluteal phase as a criterion of a potentially fertile cycle (“ovulation”) derived from treated and untreated conception cycles. Fertil Steril. 1982;37(3):355–360. [DOI] [PubMed] [Google Scholar]
- 24. Young SL, Savaris RF, Lessey BA, Sharkey AM, Balthazar U, Zaino RJ, Sherwin RA, Fritz MA. Effect of randomized serum progesterone concentration on secretory endometrial histologic development and gene expression. Hum Reprod. 2017;32(9):1903–1914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Filicori M, Butler JP, Crowley WF Jr. Neuroendocrine regulation of the corpus luteum in the human: evidence for pulsatile progesterone secretion. J Clin Invest. 1984;73(6):1638–1647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Beckers NG, Macklon NS, Eijkemans MJ, Ludwig M, Felberbaum RE, Diedrich K, Bustion S, Loumaye E, Fauser BC. Nonsupplemented luteal phase characteristics after the administration of recombinant human chorionic gonadotropin, recombinant luteinizing hormone, or gonadotropin-releasing hormone (GnRH) agonist to induce final oocyte maturation in in vitro fertilization patients after ovarian stimulation with recombinant follicle-stimulating hormone and GnRH antagonist cotreatment. J Clin Endocrinol Metab. 2003;88(9):4186–4192. [DOI] [PubMed] [Google Scholar]
- 27. Fauser BC, Devroey P. Reproductive biology and IVF: ovarian stimulation and luteal phase consequences. Trends Endocrinol Metab. 2003;14(5):236–242. [DOI] [PubMed] [Google Scholar]
- 28. Green KA, Zolton JR, Schermerhorn SM, Lewis TD, Healy MW, Terry N, DeCherney AH, Hill MJ. Progesterone luteal support after ovulation induction and intrauterine insemination: an updated systematic review and meta-analysis. Fertil Steril. 2017;107(4):924–933.e5. [DOI] [PubMed] [Google Scholar]
- 29. Quaas AM, Hansen KR. The role of steroid hormone supplementation in non-assisted reproductive technology treatments for unexplained infertility. Fertil Steril. 2016;106(7):1600–1607. [DOI] [PubMed] [Google Scholar]
- 30. Connell MT, Szatkowski JM, Terry N, DeCherney AH, Propst AM, Hill MJ. Timing luteal support in assisted reproductive technology: a systematic review. Fertil Steril. 2015;103(4):939–946.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.