Abstract
Background
International best-practice guidelines recommend completion thyroidectomy and radioiodine remnant ablation (RRA) for patients with differentiated thyroid cancer (DTC) > 4 cm or with specific risk factors. Patients with DTC < 1 cm without risk factors are recommended for lobectomy alone. Indications for aggressive surgery and RRA are less clearly defined for tumours measuring 1–4 cm. A personalised approach to decision-making is recommended.
Objectives
This study assesses therapeutic approaches to DTC as compared to the current British Thyroid Association (BTA) clinical practice guidelines. We ascertained the effect of equivocal guidance in the 1–4 cm tumour cohort on contemporary practice patterns.
Methods
Data were obtained from a prospectively maintained thyroid cancer database of patients treated for DTC in a tertiary referral centre at the University Hospital Galway. Consecutive patients attending a dedicated thyroid cancer clinic between August 2014 and August 2017 were included. Clinicopathological characteristics and management strategies were assessed.
Results
Ninety-four percent (n = 168/178) of patients were surgically managed in adherence with guidelines. A minority (n = 10) received surgery not aligned with guidelines. Ninety-seven percent (n = 172/178) of RRA treatment decisions were in accordance with guidelines. The BTA guidelines recommended a personalised decision-making approach for 18.0% (n = 32) and 44.9% (n = 80) of surgery and RRA treatment decisions, respectively. The more aggressive, treatment-driven approach was typically favoured by the multidisciplinary team, with 97% (n = 31/32) undergoing completion thyroidectomy and 100% (n = 80) proceeding to RRA.
Conclusions
Management of DTC at our institution closely adheres to contemporary clinical practice guidelines. The finding of more aggressive management in those requiring a personalised decision-making approach highlights the requirement for improved risk stratification in this cohort to rationalise management strategies.
Keywords: Differentiated thyroid cancer, Papillary cancer, Follicular cancer, Guidelines, Risk stratification, British Thyroid Association
Introduction
Thyroid cancer (TC) is the most common endocrine malignancy, accounting for 1% of all cancers, with an incidence of 162 cases per year in Ireland [1]. The National Cancer Registry Ireland reports increasing incidence of TC from 60 per year during the mid 1990s to 160 per year during the late 2000s [1]. Similar trends are reported worldwide, including the United Kingdom, the United States, and South Korea [2, 3, 4]. This observed increased incidence primarily comprises differentiated TC (DTC), without a concomitant increase in mortality, suggesting potential overdiagnosis of indolent pathology [4, 5, 6, 7]. In Ireland, the 5-year TC survival rate has improved from 71% (1994–1998) to 91.8% (2010–2014), while the 5-year DTC-specific survival is 98% [1]. Data from 51,061 DTC patients from the US Surveillance, Epidemiology, and End Results database report an overall 5-year survival of 96.5% [8]. The mainstay of TC treatment involves surgical resection with or without adjuvant radioiodine remnant ablation (RRA); it follows that overdiagnosis of DTC results in potentially avoidable morbidity arising from surgical or RRA therapies.
The key recommendations of the British Thyroid Association (BTA) 2014 guidelines for the management of thyroid cancer include diagnostic lobectomy for those with Thy3 or Thy4 fine-needle aspiration cytology (FNAC). Total thyroidectomy (TT) is advised for patients with Thy5 FNAC or with confirmed DTC following diagnostic lobectomy where tumour size exceeds 4 cm or measures any size with risk factors including multifocal, bilateral, extrathyroidal, or familial disease, and those with clinically or radiologically involved nodes or distant metastases. Lobectomy alone is deemed sufficient for patients with unifocal papillary thyroid microcarcinoma (microPTC, < 1 cm) without risk factors; these include multifocality, larger size (6–10 mm), extrathyroidal extension, poor differentiation, and desmoplastic fibrosis or an infiltrative growth pattern. There is a paucity of peer-reviewed randomised or prospective studies to support an advantage of TT over lobectomy in patients with unifocal tumours measuring 1–4 cm without risk factors. In these cases, the BTA guidelines recommend a personalised decision-making approach, which advocates a shared doctor-patient decision-making process in conjunction with multidisciplinary team input, with due consideration for recurrence risk, patient comorbidities, and personal circumstances and values [9].
The BTA recommends RRA for all patients with DTC > 4 cm or any size with gross extrathyroidal extension or distant metastases. Patients with tumours ≤1 cm without risk factors do not benefit from RRA. Personalised decision-making is advised for those with tumours measuring 1–4 cm without risk factors [9].
We aimed to describe the patterns of DTC presentation and treatment strategies and to assess the degree of adherence to clinical practice guidelines (BTA 2014) in patients treated for DTC at our institution. We further examined those patients where a personalised decision-making approach was recommended and how this subset was managed in the context of equivocal guidelines.
Materials and Methods
Data were prospectively recorded from consecutive patients attending a specialist TC clinic in a tertiary referral centre (University Hospital Galway) over 3 years (August 2014–2017). Data collection was undertaken at the time of blood and sputum sample collection for inclusion in the University Hospital Galway TC BioBank. Patient demographics, tumour histology, and staging parameters were recorded, in addition to surgical and RRA management strategies. All patients were discussed in an endocrine surgery multidisciplinary team. TC staging and FNAC were designated as per the AJCC Cancer Staging Manual (7th edition) and the UK Royal College of Pathologists thyroid cytology guidelines, respectively [10, 11]. The BTA guidelines for the management of thyroid cancer were accepted as best-practice guidelines [9]. Data recording and statistical analysis were performed using Microsoft Excel and IBM SPSS v22. Pearson's χ2 test was used to compare categorical variables, while one-way ANOVA with Tukey post hoc analyses was utilised for comparing three or more categorical, independent samples.
Results
Data were collected from 178 consecutive patients. Of these, 130 patients (73%) were female. The median age at diagnosis was 43.5 years (range 15–83 years). One hundred and fifty-two patients (85%) had papillary TC while 26 (15%) had follicular TC. The median tumour size was 26 mm (range 1–110 mm). Multifocal disease was present in 36% of patients (n = 64). Lymphovascular invasion and extracapsular extension were evident in 33% (n = 58) and 31% (n = 55) of tumours, respectively. Forty-eight percent (n = 86) had lymph nodes excised, with a mean harvest of 7.6 ± 8.5 nodes. Thirty-four patients had confirmed lymph node metastases, representing 40% of those with nodes excised and 19% of all patients. The average number of positive nodes retrieved was 4.7 ± 4.4.
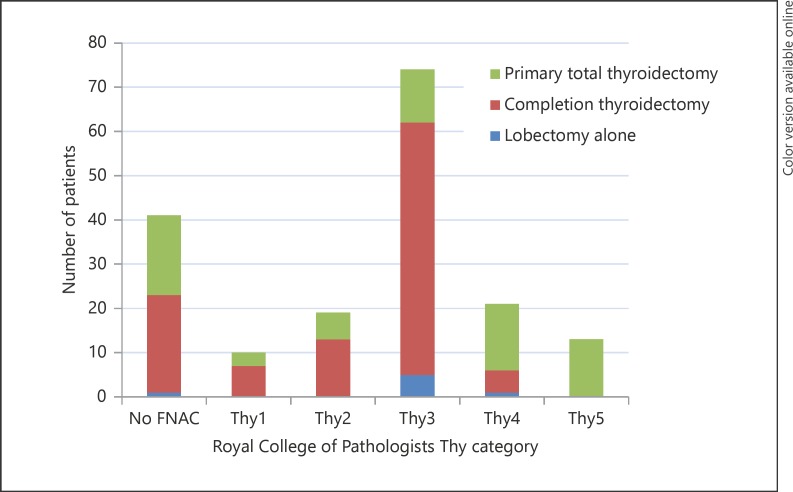
FNAC results were available for 77% of patients (n = 118) (Fig. 1). All 13 patients with Thy5 FNAC underwent primary TT. Primary TT was also performed for 3, 6, 12, and 15 patients with Thy1–4 FNACs, respectively. Of these 36 patients, 24 had features identified on preoperative imaging indicating TT (size > 4 cm, lymphadenopathy, extracapsular or bilateral disease). TT was performed for benign indications (e.g., multinodular goitre, Graves' disease) in 9 patients, while 3 patients had no definitive indication for upfront TT.
Fig. 1.
Surgical management per Royal College of Pathologists Thy category.
Surgery
Thirty-eight percent of patients (n = 67) underwent primary TT. Sixty-two percent (n = 111) had thyroid lobectomy, with 95% of those (n = 105/111) proceeding to completion thyroidectomy (Table 1). Six patients (3%) had lobectomy alone; these were more likely to be male (68%, n = 4/6) compared to those who proceeded to completion thyroidectomy (27%, n = 28/105) (p = 0.031). Tumour size > 4 cm, multifocality, and extracapsular extension were associated with an increased rate of completion thyroidectomy compared to lobectomy alone (p = 0.044, p = 0.027, and p = 0.044, respectively). No significant association was observed between histological subtype or age and surgical procedure.
Table 1.
Clinicopathological parameters based on surgical outcome
| Primary TT | Lobectomy alone | Lobectomy followed by completion thyroidectomy | |
|---|---|---|---|
| Number of patients | 67 (38%) | 6 (3%) | 105 (60%) |
| Female | 51 (76%) | 2 (33%) | 77 (73%) |
| Age, years | 46±16 | 50±23 | 46±16 |
| Preoperative FNAC Thy1 | 3 (5%) | 0 (0%) | 7 (7%) |
| Preoperative FNAC Thy2 | 6 (9%) | 0 (0%) | 13 (12%) |
| Preoperative FNAC Thy3 | 12 (18%) | 5 (83%) | 57 (54%) |
| Preoperative FNAC Thy4 | 15 (22%) | 1 (17%) | 5 (5%) |
| Preoperative FNAC Thy5 | 13 (19%) | 0 (0%) | 0 (0%) |
| No preoperative FNAC | 18 (27%) | 0 (0%) | 23 (22%) |
| Papillary (final histology) | 61 (91%) | 5 (83%) | 86 (82%) |
| Follicular (final histology) | 6 (9%) | 1 (17%) | 19 (18%) |
| Size, mm | 22 (2–110) | 5.25 (2–65) | 30 (1–110) |
| Multifocal | 26 (39%) | 0 (0%) | 38 (36%) |
| Nodal disease | 25 (37%) | 0 (0%) | 9 (9%) |
| Lymphovascular invasion | 29 (43%) | 1 (17%) | 28 (27%) |
| Extracapsular extension | 23 (34%) | 1 (17%) | 31 (30%) |
Values are presented as n (%), mean ± SD, or median (range). FNAC, fine-needle aspiration cytology; TT, total thyroidectomy.
Ninety-four percent of patients (n = 168/178) were surgically managed in strict adherence with the BTA guidelines (Table 2). Of those not aligned with the guidelines (n = 10), 1 had a tumour > 4 cm at lobectomy, but declined completion thyroidectomy. Nine patients had tumours < 1 cm without risk factors, of whom 4 had primary TT for multinodular goitre causing mass effect. Five patients had interval completion thyroidectomy following lobectomy where not indicated by guidelines, and 2 due to personal preference on a background of thyroid tumour family history; another patient had interval radiological lymphadenopathy and a recent history of metachronous laryngeal cancer. The remaining 2 patients proceeded to completion thyroidectomy based on preference alone, with age > 45 years being their only relative risk factor for recurrence. The rate of surgical management in agreement with the guidelines was not significantly affected by sex (p = 0.824), age > 45 years (p = 0.158), or histological subtype (p = 0.671).
Table 2.
Surgical management compliance with the BTA 2014 guidelines
| Patients | Compliant with BTA guidelines | |
|---|---|---|
| All patients | 178 (100%) | 168 (94%) |
| <1 cm | 27 (15%) | 18 (67%) |
| 1–4 cm | 113 (63%) | 113 (100%) |
| >4 cm | 38 (21%) | 37 (97%) |
| Multifocal | 64 (36%) | 64 (100%) |
| Lymphovascular invasion | 58 (33%) | 57 (98%) |
| Extracapsular extension | 55 (31%) | 54 (98%) |
| Age >45 years | 86 (48%) | 79 (92%) |
Values are presented as n (%). BTA, British Thyroid Association.
Personalised decision-making was recommended following thyroid lobectomy in 32 patients (Table 3); 24 patients had intermediate size tumours (1–4 cm) without risk factors, 4 had microPTC measuring 6–10 mm, while 4 with microPTC < 6 mm exhibited multifocal disease. A more aggressive, treatment-driven approach was typically favoured by patients and the multidisciplinary team, with 97% (n = 31/32) proceeding to completion thyroidectomy; 1 patient with a unifocal 15-mm papillary TC without risk factors opted for lobectomy alone.
Table 3.
Clinicopathological details for patients where the BTA recommended a personalised decision-making approach (n = 178)
| PDM recommended for surgical management (n = 32) | PDM recommended for RRA management (n = 80) | |
|---|---|---|
| Had thyroidectomy/RRA | 31 (97%) | 66 (83%) |
| Female | 25 (78%) | 66 (83%) |
| Age, years | 37±10 | 43±15 |
| Papillary cancer | 28 (88%) | 70 (88%) |
| Follicular cancer | 4 (13%) | 10 (12%) |
| Size, mm | 21 (2–40) | 22 (10–40) |
| <1 cm | 8 (25%) | 1 (1%) |
| 1–4 cm | 24 (75%) | 79 (99%) |
| >4 cm | 0 (0%) | 0 (0%) |
| Multifocal disease | 4 (all microPTC) | 30 (38%) |
Values are presented as n (%), mean ± SD, or median (range). BTA, British Thyroid Association; microPTC, papillary thyroid microcarcinoma; PDM, personalised decision-making; RRA, radioiodine remnant ablation.
Radioiodine Remnant Ablation
Eighty-two percent of patients (n = 146/178) underwent adjuvant RRA. RRA was more likely to be utilised in patients with node positivity (p = 0.002), lymphovascular invasion (p < 0.001), or extracapsular disease (p = 0.001). RRA utilisation was not affected by sex (p = 0.547), age > 45 years (p = 0.203), histological subtype type (p = 0.139), or multifocality (p = 0.067).
In relation to the BTA guidelines, 43% of patients (n = 77) had definitive indications for RRA, while 12% (n = 21) had definitive recommendations against RRA. A personalised decision-making approach was recommended for 45% of patients (n = 80), with 66 of those (83%) progressing to RRA (Table 3). Ninety-seven percent (n = 172/178) of RRA treatment decisions satisfied the 2014 BTA guidelines; 1 patient recommended for RRA due to extrathyroidal extension declined, while the remaining 6 patients received RRA outside of the BTA recommendations. None of these 6 patients had definitive RRA indications; however, 2 had weaker risk factors for recurrence, one with unilateral multifocal microPTC, the other being > 45 years old. Agreement with the BTA recommendations was not significantly affected by sex (p = 0.721), age > 45 years (p = 0.115), or histological subtype (p = 0.884). Patients with tumours measuring < 1 cm were more likely to undergo RRA management differing from the BTA recommendations (i.e., proceeding to RRA) when compared to patients in the 1–4 cm or > 4 cm subgroups (p < 0.001).
Discussion
This study assessed the DTC management patterns in an Irish tertiary referral centre, with a focus on patients with intermediate-size, low-risk tumours, where a paucity of evidence prevents guidelines from supporting a definitive therapeutic approach. DTC treatment strategies were largely in agreement with best-practice recommendations, with concordance demonstrated in 94% of surgical and 97% of RRA therapeutic decisions. Where discordance with guidelines was demonstrated, there was a tendency towards overtreatment, with 9 out of 10 surgical and 5 out of 6 RRA treatment decisions resulting in a more aggressive treatment (TT and RRA) where lobectomy and no RRA was recommended. Early studies examining the appropriate surgical management of DTC initially suggested a one size fits all approach with TT, resulting in lower recurrence rates and improved survival for all patients with DTC > 1 cm [12, 13]. More recently, recognition of relevant prognosticators has improved risk stratification such that low-risk patients with tumours > 1 cm may be treated with more selective and individualised approaches while maintaining improved outcomes [14, 15, 16, 17]. This is evidenced by incremental guideline amendments towards more conservative approaches; the 2009 American Thyroid Association (ATA) and the 2006 European Thyroid Cancer Consensus guidelines both previously recommended TT for DTC > 1 cm, while the more recent 2014 BTA and 2015 ATA guidelines now suggest lobectomy alone as an option for those with tumours 1–4 cm without risk factors. Emerging evidence also advocates active surveillance for microPTC, with interval growth over 5 years observed in < 15% of patients [18, 19]. Improved identification and classification of indolent subtypes further improves risk stratification; the encapsulated follicular variant of papillary TC has recently been re-designated as non-invasive follicular thyroid neoplasm with papillary-like nuclear features, effectively reclassifying it as non-cancerous [20].
Almost half of our cohort was subject to the equivocal BTA guidelines, whereby a personalised decision-making approach was suggested. These patients typically had intermediate-sized tumours without specific recurrence risk factors. Despite large retrospective cohort studies, the infrequency of mortality and disease recurrence events seen in this cohort poses a challenge for the development of definitive evidence-based guidelines. In the absence of specific recommendations, the personalised decision-making approach encourages consideration of factors such as patient preference, age, comorbidity, performance status, ability to engage with contralateral lobe follow-up, and the potential impact of surgical complications. Clinician preference, surgeon complication rates, and tumour parameters tending towards guideline cut-offs (e.g., size approaching 1 or 4 cm) should also be considered. Almost all patients in our cohort who were recommended for a personalised decision-making approach proceeded to the more aggressive options of completion surgery and RRA. The absence of sufficient evidence to support a definitive treatment course in these patients despite multiple large retrospective studies suggests that any benefit gained by this strategy is likely to be small [21]. Furthermore, the improved efficacy of treatments for recurrent DTC is such that the lower risk of recurrence for patients in the personalised decision-making cohort may be acceptable, given the benefit of avoiding a second surgery and exposure to RRA. Benefits gained by the more aggressive options may be outweighed by both physical and psychological morbidity. The impact of extended patient waiting times, from initial diagnostic imaging and FNAC to thyroid lobectomy, subsequent completion surgery, and onward to RRA, is extensive, resulting in substantial psychological morbidity [22]. Surgical complications are also considerable and include transient (8%) and permanent (2%) hyperparathyroidism, permanent (1%), transient (2%), and diplegic (0.4%) palsies of the recurrent laryngeal nerve, superior thyroid nerve injury (4%), and dysphagia (1%) [23]. There are also financial implications; thyroid lobectomy costs EUR 5,277 per patient in Europe, including presurgical workup, follow-up, and management of complications over 12 months [24], in addition to the lifelong cost of thyroxine replacement, follow-up, and monitoring associated with TT. In New South Wales, Australia, the increased volume of DTC treatments from 2002 to 2012, for a population of 7.5 million, has cost an additional AUD 18,600,000 in surgery-related healthcare expenditure [25].
RRA is also associated with significant side effects. Twenty percent of patients experience nausea, taste and smell impairment, or sialadenitis. More significant complications include impairment of haematopoiesis and gonadal function and increased risk for second primary malignancies, both solid and haematological. A dose of 100 mCi (3.7 GBq) of radioiodine has been estimated to result in an extra 56 malignancies per 10,000 patients over 10 years [24], while Rubino et al. [26] observed up to 30% dose-dependent increased risk for second primary malignancies following RRA.
While DTC has a relatively good prognosis compared to other malignancies, there remains a recurrence risk of 5–30%, and approximately 10% of patients die of this cancer [8, 27, 28, 29]. Improved risk stratification is warranted to identify patients at risk of mortality and recurrence. Multiple risk factor assessment tools exist for estimating DTC mortality. Prognostication systems include the AJCC/UICC TNM system (Tumour Nodes Metastases), AMES (Age, Metastases, Extent, Size), MACIS (Metastases, Age, Completeness of resection, Extrathyroidal, Size), EORTC (European Organisation for Research and Treatment of Cancer methodology), and AGES (Age, Grade, Extent, Size) [30, 31]. These prognostication systems, using traditional demographic and staging parameters and focusing predominantly on mortality rather than recurrence risk, have largely failed to guide management in a large subset of patients, as demonstrated by the equivocal BTA 2014 guidelines for low-risk intermediate-size DTC.
Advances in molecular medicine have improved the understanding of DTC carcinogenesis and risk indicators. Molecular markers including gene expression profiles, somatic gene alterations, and circulating biomarkers provide improved indices for diagnosis and prognostication [32]. Alterations in the MAPK and PI3K-AKT major signalling pathways have recently been elucidated as primary pathogenetic events in DTC carcinogenesis [33]. The 2015 ATA guidelines now advocate consideration of BRAFV600E proto-oncogene mutation status, if known, in their modified risk stratification system, although testing is not routinely advocated. The guidelines also acknowledge other gene mutations and rearrangements such as BRAF, TERT, TP53, RAS, or PAX8/PPARγ, although these are not recommended for routine testing [34]. Rather than assessing for individual high-impact mutations, commercially available risk assessment tools analyse panels of mutations, each with smaller odds ratios for recurrence and mortality, but with promising overall accuracy. These include the Afirma Gene Expression Classifier and the ThyroSeq Next-Generation Sequencing panel. These adjuncts have demonstrated encouraging results in the risk stratification of patients with indeterminate FNAC where diagnostic lobectomy is frequently required [35, 36]. In correctly identifying DTC from FNAC samples, ThyroSeq v2.1 reports a sensitivity of 91% (95% CI 79–100) and a specificity of 92% (95% CI 86–98) [36]. This application of molecular risk stratification may help reduce the number of diagnostic thyroid lobectomies undertaken; over one-third of the patients in our cohort had initial Thy3 FNAC results, with three-quarters of these requiring completion thyroidectomy. However, while multiple studies have evaluated the utility of molecular testing in patients with indeterminate thyroid nodules, there is a paucity of research examining the use of such markers in surgical and RRA management decisions after thyroid lobectomy for DTC [32, 37].
Conclusions
DTC management in our cohort exhibited high levels of adherence to internationally recognised best-practice guidelines. Where surgical and RRA therapeutic decisions did not satisfy the guidelines, more aggressive management approaches were usually adopted.
A large proportion of patients are subject to a personalised decision-making approach, owing to a lack of conclusive high-level evidence to guide management. A tendency towards more aggressive surgical and RRA intervention was observed in this group.
These findings highlight the requirement for improved risk stratification to rationalise management strategies and avoid overtreatment of patients who fall into indeterminate-risk treatment groups.
Statement of Ethics
All patients provided informed written consent, which was ethically approved by the University Hospital Galway research ethics committee.
Disclosure Statement
The authors declare no conflicts of interest.
Acknowledgements
This work was completed as part of an MD thesis and was supported by Breast Cancer Research at the Lambe Institute for Translational Research, Galway, Ireland (no specific grant number).
References
- 1.National Cancer Registry Ireland Cancer Trends No 16. Cancer of the thyroid. Cork, National Cancer Registry. 2012 [Google Scholar]
- 2.Dos Santos Silva I, Swerdlow AJ. Thyroid cancer epidemiology in England and Wales: time trends and geographical distribution. Br J Cancer. 1993;67:330–340. doi: 10.1038/bjc.1993.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Enewold L, Zhu K, Ron E, Marrogi AJ, Stojadinovic A, Peoples GE, Devesa SS. Rising thyroid cancer incidence in the United States by demographic and tumor characteristics, 1980–2005. Cancer Epidemiol Biomarkers Prev. 2009;18:784–791. doi: 10.1158/1055-9965.EPI-08-0960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ahn HS, Kim HJ, Welch HG. Korea's thyroid-cancer “epidemic” - screening and overdiagnosis. N Engl J Med. 2014;371:1765–1767. doi: 10.1056/NEJMp1409841. [DOI] [PubMed] [Google Scholar]
- 5.Wang CC, Tsai TL, Hsing CY, Wu SH. In reference to The increasing incidence of small thyroid cancers: Where are the cases coming from? Laryngoscope. 2012;122:1181. doi: 10.1002/lary.22418. author reply 1182. [DOI] [PubMed] [Google Scholar]
- 6.Jegerlehner S, Bulliard JL, Aujesky D, Rodondi N, Germann S, Konzelmann I, Chiolero A. Overdiagnosis and overtreatment of thyroid cancer: a population-based temporal trend study. PLoS One. 2017;12:e0179387. doi: 10.1371/journal.pone.0179387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vaccarella S, Franceschi S, Bray F, Wild CP, Plummer M, Dal Maso L. Worldwide thyroid-cancer epidemic? The increasing impact of overdiagnosis. N Engl J Med. 2016;375:614–617. doi: 10.1056/NEJMp1604412. [DOI] [PubMed] [Google Scholar]
- 8.Shi RL, Qu N, Liao T, Wei WJ, Wang YL, Ji QH. The trend of age-group effect on prognosis in differentiated thyroid cancer. Sci Rep. 2016;6:27086. doi: 10.1038/srep27086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Perros P, Boelaert K, Colley S, Evans C, Evans RM, Gerrard Ba G, Gilbert J, Harrison B, Johnson SJ, Giles TE, Moss L, Lewington V, Newbold K, Taylor J, Thakker RV, Watkinson J, Williams GR, British Thyroid Association Guidelines for the management of thyroid cancer. Clin Endocrinol (Oxf) 2014;81((suppl 1)):1–122. doi: 10.1111/cen.12515. [DOI] [PubMed] [Google Scholar]
- 10.Edge SB, Compton CC. The American Joint Committee on Cancer: the 7th Edition of the AJCC cancer staging manual and the future of TNM. Ann Surg Oncol. 2010;17:1471–1474. doi: 10.1245/s10434-010-0985-4. [DOI] [PubMed] [Google Scholar]
- 11.Cross P, Chandra A, Giles T, Liverpool R, Johnson S, Kocjan G, Poller D, Stephenson T. Guidance on the reporting of thyroid cytology specimens. 2016 Jan; http://ukeps.com/docs/thyroidfna.pdf. [Google Scholar]
- 12.Mazzaferri EL, Young RL, Oertel JE, Kemmerer WT, Page CP. Papillary thyroid carcinoma: the impact of therapy in 576 patients. Medicine (Baltimore) 1977;56:171–196. [PubMed] [Google Scholar]
- 13.Bilimoria KY, Bentrem DJ, Ko CY, Stewart AK, Winchester DP, Talamonti MS, Sturgeon C. Extent of surgery affects survival for papillary thyroid cancer. Ann Surg. 2007;246:375–381. doi: 10.1097/SLA.0b013e31814697d9. discussion 381–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vaisman F, Shaha A, Fish S, Michael Tuttle R. Initial therapy with either thyroid lobectomy or total thyroidectomy without radioactive iodine remnant ablation is associated with very low rates of structural disease recurrence in properly selected patients with differentiated thyroid cancer. Clin Endocrinol (Oxf) 2011;75:112–119. doi: 10.1111/j.1365-2265.2011.04002.x. [DOI] [PubMed] [Google Scholar]
- 15.Nixon IJ, Ganly I, Patel SG, Palmer FL, Whitcher MM, Tuttle RM, Shaha A, Shah JP. Thyroid lobectomy for treatment of well differentiated intrathyroid malignancy. Surgery. 2012;151:571–579. doi: 10.1016/j.surg.2011.08.016. [DOI] [PubMed] [Google Scholar]
- 16.Matsuzu K, Sugino K, Masudo K, Nagahama M, Kitagawa W, Shibuya H, Ohkuwa K, Uruno T, Suzuki A, Magoshi S, Akaishi J, Masaki C, Kawano M, Suganuma N, Rino Y, Masuda M, Kameyama K, Takami H, Ito K. Thyroid lobectomy for papillary thyroid cancer: long-term follow-up study of 1,088 cases. World J Surg. 2014;38:68–79. doi: 10.1007/s00268-013-2224-1. [DOI] [PubMed] [Google Scholar]
- 17.Adam MA, Pura J, Goffredo P, Dinan MA, Hyslop T, Reed SD, Scheri RP, Roman SA, Sosa JA. Impact of extent of surgery on survival for papillary thyroid cancer patients younger than 45 years. J Clin Endocrinol Metab. 2015;100:115–121. doi: 10.1210/jc.2014-3039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ito Y, Miyauchi A, Oda H. Low-risk papillary microcarcinoma of the thyroid: a review of active surveillance trials. Eur J Surg Oncol. 2018;44:307–315. doi: 10.1016/j.ejso.2017.03.004. [DOI] [PubMed] [Google Scholar]
- 19.Tuttle RM, Fagin JA, Minkowitz G, Wong RJ, Roman B, Patel S, Untch B, Ganly I, Shaha AR, Shah JP, Pace M, Li D, Bach A, Lin O, Whiting A, Ghossein R, Landa I, Sabra M, Boucai L, Fish S, Morris LGT. Natural history and tumor volume kinetics of papillary thyroid cancers during active surveillance. JAMA Otolaryngol Head Neck Surg. 2017;143:1015–1020. doi: 10.1001/jamaoto.2017.1442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nikiforov YE, Seethala RR, Tallini G, Baloch ZW, Basolo F, Thompson LD, Barletta JA, Wenig BM, Al Ghuzlan A, Kakudo K, Giordano TJ, Alves VA, Khanafshar E, Asa SL, El-Naggar AK, Gooding WE, Hodak SP, Lloyd RV, Maytal G, Mete O, Nikiforova MN, Nosé V, Papotti M, Poller DN, Sadow PM, Tischler AS, Tuttle RM, Wall KB, LiVolsi VA, Randolph GW, Ghossein RA. Nomenclature revision for encapsulated follicular variant of papillary thyroid carcinoma: a paradigm shift to reduce overtreatment of indolent tumors. JAMA Oncol. 2016;2:1023–1029. doi: 10.1001/jamaoncol.2016.0386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Asimakopoulos P, Nixon IJ. Surgical management of primary thyroid tumours. Eur J Surg Oncol. 2018;44:321–326. doi: 10.1016/j.ejso.2016.12.015. [DOI] [PubMed] [Google Scholar]
- 22.Eskander A, Devins GM, Freeman J, Wei AC, Rotstein L, Chauhan N, Sawka AM, Brown D, Irish J, Gilbert R, Gullane P, Higgins K, Enepekides D, Goldstein D. Waiting for thyroid surgery: a study of psychological morbidity and determinants of health associated with long wait times for thyroid surgery. Laryngoscope. 2013;123:541–547. doi: 10.1002/lary.23503. [DOI] [PubMed] [Google Scholar]
- 23.Rosato L, Avenia N, Bernante P, De Palma M, Gulino G, Nasi PG, Pelizzo MR, Pezzullo L. Complications of thyroid surgery: analysis of a multicentric study on 14,934 patients operated on in Italy over 5 years. World J Surg. 2004;28:271–276. doi: 10.1007/s00268-003-6903-1. [DOI] [PubMed] [Google Scholar]
- 24.Filetti S, Ladenson PW, Biffoni M, D'Ambrosio MG, Giacomelli L, Lopatriello S. The true cost of thyroid surgery determined by a micro-costing approach. Endocrine. 2017;55:519–529. doi: 10.1007/s12020-016-0980-z. [DOI] [PubMed] [Google Scholar]
- 25.Furuya-Kanamori L, Sedrakyan A, Onitilo AA, Bagheri N, Glasziou P, Doi SAR. Differentiated thyroid cancer: millions spent with no tangible gain? Endocr Relat Cancer. 2018;25:51–57. doi: 10.1530/ERC-17-0397. [DOI] [PubMed] [Google Scholar]
- 26.Rubino C, de Vathaire F, Dottorini ME, Hall P, Schvartz C, Couette JE, Dondon MG, Abbas MT, Langlois C, Schlumberger M. Second primary malignancies in thyroid cancer patients. Br J Cancer. 2003;89:1638–1644. doi: 10.1038/sj.bjc.6601319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hundahl SA, Fleming ID, Fremgen AM, Menck HR. A National Cancer Data Base report on 53,856 cases of thyroid carcinoma treated in the U.S., 1985–1995. Cancer. 1998;83:2638–2648. doi: 10.1002/(sici)1097-0142(19981215)83:12<2638::aid-cncr31>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 28.Mazzaferri EL, Jhiang SM. Long-term impact of initial surgical and medical therapy on papillary and follicular thyroid cancer. Am J Med. 1995;97:418–428. doi: 10.1016/0002-9343(94)90321-2. [DOI] [PubMed] [Google Scholar]
- 29.Na'ara S, Amit M, Fridman E, Gil Z. Contemporary management of recurrent nodal disease in differentiated thyroid carcinoma. Rambam Maimonides Med J. 2016;7 doi: 10.5041/RMMJ.10233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Teo KW, Yuan NK, Tan WB, Parameswaran R. Comparison of prognostic scoring systems in follicular thyroid cancer. Ann R Coll Surg Engl. 2017;99:479–484. doi: 10.1308/rcsann.2017.0072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Glikson E, Alon E, Bedrin L, Talmi YP. Prognostic factors in differentiated thyroid cancer revisited. Isr Med Assoc J. 2017;19:114–118. [PubMed] [Google Scholar]
- 32.Yip L. Molecular markers for thyroid cancer diagnosis, prognosis, and targeted therapy. J Surg Oncol. 2015;111:43–50. doi: 10.1002/jso.23768. [DOI] [PubMed] [Google Scholar]
- 33.Xing M. Molecular pathogenesis and mechanisms of thyroid cancer. Nat Rev Cancer. 2013;13:184–199. doi: 10.1038/nrc3431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Haugen BR, Alexander EK, Bible KC, Doherty GM, Mandel SJ, Nikiforov YE, Pacini F, Randolph GW, Sawka AM, Schlumberger M, Schuff KG, Sherman SI, Sosa JA, Steward DL, Tuttle RM, Wartofsky L. 2015 American Thyroid Association Management Guidelines for Adult Patients with Thyroid Nodules and Differentiated Thyroid Cancer: The American Thyroid Association Guidelines Task Force on Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid. 2016;26:1–133. doi: 10.1089/thy.2015.0020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kargi AY, Bustamante MP, Gulec S. Genomic profiling of thyroid nodules: current role for ThyroSeq next-generation sequencing on clinical decision-making. Mol Imaging Radionucl Ther. 2017;26((suppl 1)):24–35. doi: 10.4274/2017.26.suppl.04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nikiforov YE, Carty SE, Chiosea SI, Coyne C, Duvvuri U, Ferris RL, Gooding WE, LeBeau SO, Ohori NP, Seethala RR, Tublin ME, Yip L, Nikiforova MN. Impact of the multi-gene ThyroSeq next-generation sequencing assay on cancer diagnosis in thyroid nodules with atypia of undetermined significance/follicular lesion of undetermined significance cytology. Thyroid. 2015;25:1217–1223. doi: 10.1089/thy.2015.0305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yip L, Sosa JA. Molecular-directed treatment of differentiated thyroid cancer: advances in diagnosis and treatment. JAMA Surg. 2016;151:663–670. doi: 10.1001/jamasurg.2016.0825. [DOI] [PubMed] [Google Scholar]