Figure 1.
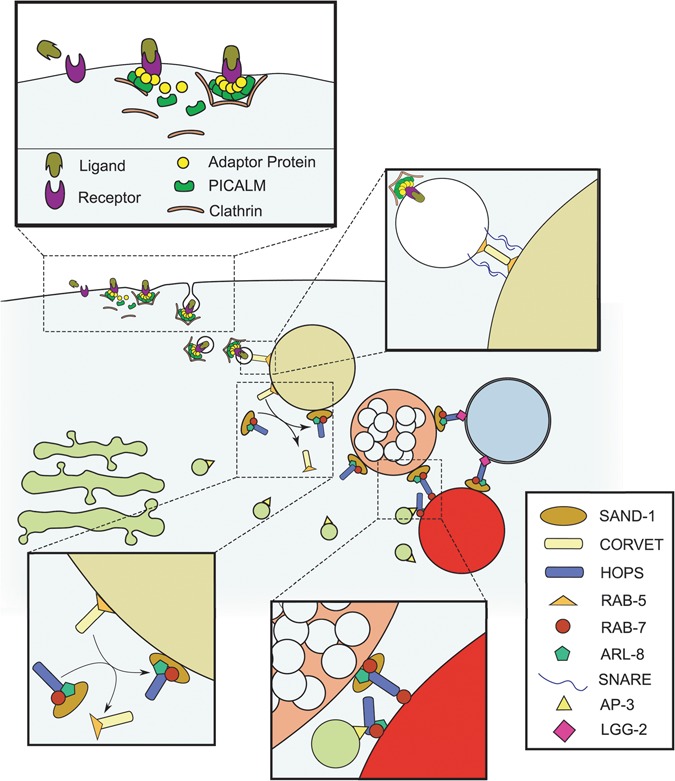
The endolysosomal trafficking system. Schematic diagram representing the proteins discussed and/or examined in this investigation is shown. Adaptor proteins stabilize the recruitment of clathrin to a ligand-receptor complex to stimulate its internalization by endocytosis. This process is facilitated by the PICALM/UNC-11 protein. Subsequently, internalized vesicles are trafficked to the EE, where SNARE-mediated tethering and fusion is facilitated by the CORVET complex, along with RAB-5. As the EE accumulates delivered cargoes, the increasing abundance of phosphatidylinositol-3-phosphate in the EE membrane stabilizes the recruitment of the SAND-1/Mon1-Ccz1 complex, which brings with it the HOPS complex (which includes VPS-41), RAB-7 and ARL-8. This process coincides with the progressive acidification of the endosome, in addition to the accumulation of intraluminal vesicles that define the LE. The HOPS complex tethers the LE with the lysosome through RAB-7 or ARL-8 and stabilizes the recruitment of SNARE proteins for homotypic fusion. On the lysosomal surface, the HOPS complex also mediates the tethering and fusion of autophagosomes with the lysosome through its interactions with LC3/LGG-2 and RAB-7 or ARL-8. Additionally, the AP3-interacting domain of VPS-41 of the HOPS complex on the lysosomal surface can be made accessible to AP3 coating the vesicles arriving from the trans-Golgi.
