Figure 1.
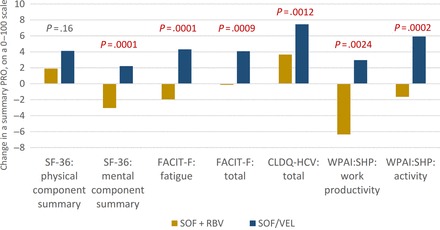
End-of-treatment changes in summary patient-reported outcomes (PROs) from patients' own baseline levels in patients treated with sofosbuvir (SOF)/velpatasvir and SOF + ribavirin regardless of duration. P values reflect differences between the 2 treatment groups. Changes exceeding 2 points were also statistically significant (P < .05) when compared with zero change (ie, no change from patient's own baseline PRO level). Abbreviations: CLDQ-HCV, chronic liver disease questionnaire-hepatitis C virus version; FACIT-F, functional assessment of chronic illness therapy-fatigue; PRO, patient-reported outcome; RBV, ribavirin; SF-36, short-form-36; SOF, sofosbuvir; VEL, velpatasvir; WPAI:SHP, work productivity activity index:specific health problem.
