Figure 2.
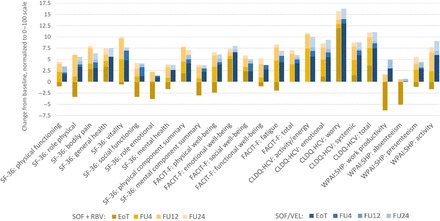
Post-treatment incremental changes in patient-reported outcomes (PROs) in patients treated with sofosbuvir (SOF) + ribavirin and SOF/velpatasvir . At end of treatment, P < .05 between the 2 regimens for all but 6 PROs (SF-36: bodily pain, general health, physical component summary; FACIT-F: emotional well-being, social well-being; CLDQ-HCV: worry). At post-treatment follow-up (FU)4, P < .05 for only 3 PROs (SF-36: mental component summary; FACIT-F: functional well-being; CLDQ-HCV: systemic). At FU12 and FU24, all P > .05 between the 2 regimens. Abbreviations: CLDQ-HCV, chronic liver disease questionnaire-hepatitis C virus version; EoT, end of treatment; FACIT-F, functional assessment of chronic illness therapy-fatigue; FU, post-treatment follow-up; RBV, ribavirin; SF-36, short-form-36; SOF, sofosbuvir; VEL, velpatasvir; WPAI:SHP, work productivity activity index:specific health problem.
