Abstract
Previous studies have reported that telomere length in peripheral blood leukocytes can predict the clinical outcome of several cancers. However, whether leukocyte telomere length is associated with the prognosis of hepatocellular carcinoma (HCC) remains to be determined. In this study, relative telomere length (RTL) in peripheral blood leukocytes was measured using a real-time PCR-based method for 269 HCC patients treated with transarterial chemoembolization (TACE) from two independent hospitals. The association between RTL and the overall survival (OS) of HCC was analyzed. The immunological function of the HCC patients with different leukocyte RTLs was evaluated. Multivariate analyses indicated that long leukocyte RTL was significantly associated with poor OS of HCC patients, with a hazard ratio of 2.04 (95% confidence interval, 1.46–2.86; P < 0.001). Kaplan–Meier analyses showed a significant difference of median survival time between patients with long and short RTL (log rank P < 0.001). Fluorescence-activated cell sorting analyses showed that the long RTL group had a significantly increased percentage of CD4 + CD25 + FOXP3 + Treg in CD4 + T cells compared with short RTL group ( P = 0.002). In conclusion, our results suggest that leukocyte RTL may serve as an independent prognostic marker for HCC patients treated with TACE.
Introduction
Telomeres are specialized hexameric (TTAGGG) n repeat sequence located at eukaryotic chromosomal ends. Telomeres protect chromosome ends from degradation, end-to-end fusion and atypical recombination. Chromosome instability induced by telomere dysfunction has been found to contribute to the development and progression of many malignancies ( 1 ). Recent studies have focused on the role of telomere length in the prediction of cancer risk and prognosis. Some studies showed that shorter leukocyte relative telomere length (RTL) was associated with higher risk of gastric, lung and renal cancers ( 2–4 ), whereas several other reports demonstrated that longer leukocyte RTL was linked to higher risk of breast cancer, melanoma and non-Hodgkin lymphoma ( 5–7 ). In addition, two studies have evaluated the association between leukocyte RTL and cancer prognosis and reported that patients with longer leukocyte RTL had worse overall survival (OS) of breast cancer ( 8 ) and kidney cancer ( 9 ). Taken together, these data suggest a potentially important role of telomere length in the prediction of cancer risk and prognosis.
Hepatocellular carcinoma (HCC) is one of the most common malignancies worldwide ( 10 ). Only ∼30% of patients are eligible to receive surgery. The current standard of care for unresectable HCC patients is local–regional therapy with transarterial chemoembolisation (TACE) ( 11 ). Treatment outcomes of TACE are affected by multiple baseline clinical factors, such as liver function, performance status and tumor stage and vary significantly among HCC patients. However, as yet very few reports have been published on the identification of biomarkers for the prediction of HCC patient prognosis after TACE treatment. To improve the survival of TACE-treated HCC patients, it is necessary to develop novel and clinically applicable biomarkers to predict patient clinical outcomes and tailor the treatments based on the individual profile of each patient.
Previous studies have suggested that telomere shortening might contribute to the development of HCC ( 12 ). HCC patients with longer telomeres had worse survival compared with those with short telomeres ( 13 ). However, these studies have been focused on the measurement of the telomere length in cancer tissues rather than the easily accessible blood sample. In a recent study, we found that long leukocyte telomere length was associated with an increased risk of developing HCC ( 14 ). However, whether there is an association between telomere length and HCC clinical outcome has not been evaluated.
In the present study, we sought to assess whether leukocyte telomere length can be used to predict the OS of HCC patients receiving the TACE treatment. We also designed the experiment to further explore the potential biological mechanism underlying the effect of telomere length on HCC prognosis. To the best of our knowledge, this is the first study to investigate the role of leukocyte telomere length in predicting HCC prognosis.
Materials and methods
Study population
Originally, 420 unresectable patients with primary HCC were recruited into an ongoing epidemiological study between January 2008 and November 2010 at the Departments of Interventional Radiology in the Tangdu Hospital (Hospital 1, n = 111) affiliated with the Fourth Military Medical University in Xi’an and the Eastern Hepatobiliary Surgery Hospital (Hospital 2, n = 309) affiliated with the Secondary Military Medical University in Shanghai. All cases had no previous history of other cancers or cancer-related treatments. There was no restriction on age, sex or disease stage for patient recruitment. After recruitment, all patients received TACE as the first-line treatment. Among these patients, 305 (72.6%) received TACE-only, whereas the remaining 115 (27.4%) received TACE and other non-surgical treatments, such as radio frequency ablation, percutaneous ethanol injection, gamma knife radiosurgery and/or radioimmunotherapy. To rule out the potential confounding effect of different treatment protocols, we only included HCC patients with the mono-treatment of TACE in this study. We restricted our analysis to Han Chinese patients since they represented 99% of our study population. Of the 305 patients received TACE-only, 33 patients were excluded due to incomplete clinical or follow-up information and 3 patients were excluded due to poor DNA quality. Finally, a total of 269 patients were used for the analysis on the association between RTL and the OS of HCC in this study. Moreover, 17 additional HCC patients were enrolled for the detection of T-cell immunophenotypes between 2 and 16 August 2011 at the Department of Interventional Radiology in Tangdu Hospital. All participants were successfully assayed for telomere length. This study was approved by the related Institutional Review Boards in Xi’an and Shanghai, and the signed informed consent was obtained from each participant.
Demographic and clinical data
Demographic and personal data were collected through an in-person interview using a standardized questionnaire. Before the first TACE treatment, 5 ml of venous blood from each patient was drawn into coded sodium citrate anticoagulant tubes and delivered to the laboratory for analysis. Detailed clinical information was collected through medical review or consultation with treating physicians. At 3 month intervals, follow-up information on death of patients was updated by a trained clinical specialist through on-site interview, direct calling or medical chart review. The latest follow-up data in this analysis were obtained in June 2011. Among the currently enrolled patients for prognosis analysis, the percentage of patient lost during follow-up was 4.8% ( n = 13).
Measurement of RTL
The RTL was measured on extracted genomic DNA in duplicates using a real-time PCR-based method as described in our previous study ( 14 ). Briefly, this measurement includes two main steps. First, the ratio of the telomere repeats copy number (T) and the single copy gene (human globulin) copy number (S) was determined for each sample using standard curves. The derived T/S ratio was proportional to the RTL. Second, the ratio of each sample was normalized by a calibrator DNA sample to standardize between different PCR runs. The calibrated T/S ratio was then used as the measurement of RTL in this study. The same negative and positive controls, a calibrator DNA and samples for constructing a standard curve were included on each plate. The data were analyzed using the Stratagene Mx3005p QPCR software. The R2 correlation coefficient for each standard curve was ≥0.99, with acceptable standard deviations set at 0.25 (for the Ct values). If the data was found to be out of the acceptable range of the standard curve, the sample test was repeated.
Immunophenotype analysis of T cell by flow cytometry
Flow cytometry was used to analyze the T-cell immunophenotypes, including CD3, CD4, CD8, CD25 and FOXP3, as described previously ( 15 ). Briefly, peripheral blood mononuclear cells (PBMCs) were first isolated from whole blood samples by density gradient centrifugation over Ficoll-Hypaque (Amersham Pharmacia Biotech, NJ). Phenotypic analysis was then carried out by staining PBMC with the following panel of antibodies: APC-CD3, APC-CD8 (BD Biosciences, San Diego, CA), (Alexa Fluor® 488)-FOXP3, (PE-Cy5)-CD4 and (PE)-CD25 (BioLegend, San Diego, CA). Appropriate isotype controls were included for each sample. All detections were performed on a FACScan flow cytometer equipped with Expo32 software (Beckman Coulter). Finally, the percentage of Treg (CD4 + CD25 + FOXP3 + ) in CD4 + T cells, the percentage of T cells in PBMCs and the ratio of CD4 + /CD8 + T cells were determined for each sample.
Statistical analysis
All statistical analyses were performed using the SPSS 16.0 statistical package for Microsoft Windows (SPSS, Chicago, IL). Wilcoxon rank sum test was used to evaluate the differences in RTL between different subgroups stratified by the demographic and clinical characteristics of the patients. Pearson’s correlation coefficient was used to determine the correlation between RTL and age in all patients. Cumulative survival time was calculated using the Kaplan–Meier procedure. The difference on cumulative survival between different groups was analyzed using a log-rank test. The univariate and multivariate analyses based on the Cox proportional hazards regression model were performed to identify the prognostic factors for OS. All tests in this study were two-sided and P ≤0.05 was considered statistically significant.
Results
Characteristics of the study population
Demographic and clinical characteristics of the 269 HCC patients from two hospitals are summarized in Table I . There was a patient heterogeneity between the two hospitals with respect to certain tumor characteristics, such as background liver cirrhosis, tumor size, number of tumors, tumor-node-metastasis (TNM) stage and Child–Pugh score. Hospital 2 ( n = 203) had a higher percentage of patients in advanced stages than Hospital 1 ( n = 66). In addition, median age was significantly older for patients from Hospital 1 (57 years) than those from Hospital 2 (53 years) ( P = 0.031). Such heterogeneity may help to ensure that molecular predictors have real-world applicability across heterogeneous population of patients. For the total of 269 patients, the median age at the time of HCC diagnosis was 54 years (range, 31–80 years). The majority of patients (88.5%) were males and 238 (88.5%) of all patients had hepatitis B virus infection. About 67.3% (181) of patients had liver cirrhosis and almost all patients (91.1%) had a Child–Pugh score A. There were 71.1% (191) of patients with tumor size of ≥5 cm, 52.8% (142) with multiple tumor nodules (≥2), 28.6% (77) with portal vein thrombus (PVT) and 56.5% (152) with significantly increased serum alpha-fetoprotein (AFP) (≥200 μg/l). According to the sixth edition of TNM Classification of International Union Against Cancer, the percentage (number) of patients with TNM stage I, II, III and IV was 29.0 (78), 11.9 ( 16 ), 53.9 (145) and 5.2% (14), respectively. The median follow-up time was 16.7 months (ranging from 3.0 to 42.1 months) for all patients, 18.5 months (ranging from 3.0 to 42.1 months) for patients from Hospital 1 and 14.0 months (ranging from 3.0 to 40.0 months) for patients from Hospital 2. At last follow-up, there were 155 deaths (50 from Hospital 1 and 105 from Hospital 2) among which 152 (98.1%) died due to hepatic decompensation and/or tumor progression, whereas 3 (1.9%) were due to other causes. The median survival time was 13.0 months for all patients, 16.0 months for patients from Hospital 1 and 12.0 months for patients from Hospital 2.
Table I.
Demographic and clinical characteristics of HCC patients with TACE treatment
| Variables | Hospital 1 ( n = 66) | Hospital 2 ( n = 203) | P value | Total ( n = 269) |
| Number (%) | Number (%) | Number (%) | ||
| Sex | ||||
| Male | 62 (93.9%) | 176 (86.7%) | 238 (88.5%) | |
| Female | 4 (6.1%) | 27 (13.3%) | 0.125 a | 31 (11.5%) |
| HBsAg | ||||
| Positive | 58 (87.9%) | 180 (88.7%) | 238 (88.5%) | |
| Negative | 8 (12.1%) | 23 (11.3%) | 0.827 a | 31 (11.5%) |
| Liver cirrhosis | ||||
| Yes | 33 (50.0%) | 148 (72.9%) | 181 (67.3%) | |
| No | 33 (50.0%) | 55 (27.1%) | <0.001 a | 88 (32.7%) |
| Tumor size (cm) | ||||
| <5 | 27 (40.9%) | 51 (25.1%) | 78 (28.9%) | |
| ≥5 | 39 (59.1%) | 152 (74.9%) | 0.019 a | 191 (71.1%) |
| Number of tumors | ||||
| Single | 46 (69.7%) | 81 (39.9%) | 127 (47.2%) | |
| Multiple | 20 (30.3%) | 122 (60.1%) | <0.001 a | 142 (52.8%) |
| PVT | ||||
| Yes | 13 (19.7%) | 64 (31.5%) | 77 (28.6%) | |
| No | 53 (80.3%) | 139 (68.5%) | 0.084 a | 192 (71.4%) |
| TNM stage | ||||
| I | 34 (51.6%) | 44 (21.7%) | 78 (29.0%) | |
| II | 9 (13.6%) | 23 (11.3%) | 32 (11.9%) | |
| III | 23 (34.8%) | 122 (60.1%) | 145 (53.9%) | |
| IV | 0 (0.0%) | 14 (6.9%) | <0.001 b | 14 (5.2%) |
| Child–Pugh score | ||||
| A | 50 (75.8%) | 195 (96.1%) | 245 (91.1%) | |
| B | 16 (24.2%) | 8 (3.9%) | <0.001 a | 24 (8.9%) |
| Serum AFP (μg/l) | ||||
| <200 | 29 (43.9%) | 88 (43.3%) | 117 (43.5%) | |
| ≥200 | 37 (56.1%) | 115 (56.7%) | 1.000 a | 152 (56.5%) |
| Death | ||||
| Yes | 50 (75.8%) | 105 (51.7%) | 155 (57.6%) | |
| No | 16 (24.2%) | 98 (48.3%) | 0.001 a | 114 (42.4%) |
| Age (years), mean ± SD | 57 ± 11.1 | 53 ± 11.8 | 0.031 c | 54 ± 11.7 |
| RTL, median (range) | 0.57 (0.32–1.23) | 0.63 (0.17–1.80) | 0.027 d | 0.60 (0.17–1.80) |
The P values were calculated using a chi-square test.
The P values were calculated using a chi-square test for TNM stage (I + II)/(III + IV).
The P values were calculated using an unpaired student’s t test.
The P value was calculated using a Wilcoxon rank sum test.
Relationship between RTL and HCC patient characteristics
The median RTL of all patients was 0.60 (ranging from 0.17 to 1.80) ( Table I ). There was a significantly shorter median RTL in patients from Hospital 1 (median = 0.57, range = 0.32–1.23) than those from Hospital 2 (median = 0.63, range = 0.17–1.80) ( P = 0.027). In stratified analyses of all patients, there was a significant difference in RTL between young (<54 years) and old patients (≥54 years) ( P = 0.005) ( Table II ). Further analysis showed a significant inverse correlation between RTL and age ( r = −0.362; P < 0.001) for all patients ( Supplementary Figure 1 is available at Carcinogenesis Online). However, RTL was not associated with sex, HBsAg, tumor size, liver cirrhosis, number of tumors, PVT, TNM stage, Child–Pugh score and AFP level ( Table II ).
Table II.
Comparison of RTL between different subgroups of all patients stratified by host characteristics
| Variables | RTL, median (range) | P value |
| Age (years) | ||
| <54 | 0.63 (0.17–1.80) | 0.005 |
| ≥54 | 0.54 (0.17–1.79) | |
| Sex | ||
| Male | 0.63 (0.21–1.80) | 0.555 |
| Female | 0.60 (0.17–1.67) | |
| HBsAg | ||
| Positive | 0.60 (0.17–1.80) | 0.642 |
| Negative | 0.62 (0.30–1.79) | |
| Liver cirrhosis | ||
| Yes | 0.60 (0.17–1.67) | 0.933 |
| No | 0.59 (0.22–1.80) | |
| Tumor size (cm) | ||
| <5 | 0.64 (0.17–1.79) | 0.091 |
| ≥5 | 0.58 (0.17–1.80) | |
| Number of tumors | ||
| Single | 0.58 (0.17–1.80) | 0.319 |
| Multiple | 0.62 (0.17–1.67) | |
| PVT | ||
| Yes | 0.58 (0.17–1.45) | 0.454 |
| No | 0.61 (0.17–1.80) | |
| TNM stage | ||
| I + II | 0.61 (0.17–1.80) | 0.277 |
| III + IV | 0.59 (0.17–1.67) | |
| Child–Pugh score | ||
| A | 0.60 (0.17–1.80) | 0.713 |
| B | 0.58 (0.26–1.14) | |
| Serum AFP (μg/l) | ||
| <200 | 0.59 (0.17–1.80) | 0.642 |
| ≥200 | 0.62 (0.19–1.67) |
The P values were calculated using a Wilcoxon rank sum test.
Prognostic significance of clinical characteristics
Cox proportional hazards regression model was used to identify prognostic factors of OS. First, univariate analyses showed that large tumor size (≥5 cm) [2.98 (1.73–5.37); P < 0.001], positive PVT [2.37 (1.70–3.30); P < 0.001], Child–Pugh score B [hazard ratio (95% confidence interval, CI), 1.75 (1.10–2.77); P = 0.018], increased serum AFP (≥200 μg/l) [1.96 (1.40–2.74); P < 0.001] and advanced TNM stage (III–IV) [2.51 (1.77–3.55); P < 0.001] predicted poor clinical outcome of all patients ( n = 269). Sex, age, liver cirrhosis and number of tumors were not associated with OS ( P = 0.179, P = 0.139, P = 0.932 and P = 0.580, respectively) ( Supplementary Table 1 is available at Carcinogenesis Online). Furthermore, multivariate analysis revealed that Child–Pugh score [hazard ratio (95% CI), 1.93 (1.21–3.09); P = 0.006], tumor size [2.40 (1.57–3.66); P < 0.001], PVT [2.14 (1.51–3.02); P < 0.001] and AFP [1.84 (1.31–2.60); P = 0.001] remained as independent prognostic predictors for OS in all patients ( Table III ). Because tumor staging was based on tumor size, tumor number and vascular invasion, we also conducted the multivariate Cox model with tumor stage and obtained similar results (data not shown). Similar results were obtained when univariate and multivariate analyses were respectively performed for patients from Hospital 1 ( n = 66) and Hospital 2 ( n = 203), except for the Child–Pugh score that was not associated with OS in patients from Hospital 1.
Table III.
Multivariate analyses of factors associated with OS
| Variables | Hospital 1 ( n = 66) | Hospital 2 ( n = 203) | Total ( n = 269) | |||
| HR (95% CI) | P value | HR (95% CI) | P value | HR (95% CI) | P value | |
| Tumor size (cm) (≥5 versus <5) | 3.13 (1.62–5.99) | <0.001 | 2.31 (1.27–4.20) | 0.006 | 2.40 (1.57–3.66) | <0.001 |
| PVT (yes versus no) | 2.51 (1.21–5.21) | 0.01 | 1.94 (1.30–2.89) | 0.001 | 2.14 (1.51–3.02) | <0.001 |
| Child–Pugh score (B versus A) | 1.62 (0.81–2.86) | 0.101 | 2.69 (1.23–5.85) | 0.013 | 1.93 (1.21–3.09) | 0.006 |
| AFP (μg/l) (≥200 versus <200) | 2.11 (1.12–4.00) | 0.021 | 1.78 (1.18–2.72) | 0.007 | 1.84 (1.31–2.60) | 0.001 |
| RTL (long versus short) | 1.64 (1.33–2.72) | 0.026 | 2.59 (1.71–3.92) | <0.001 | 2.04 (1.46–2.86) | <0.001 |
HR, hazard ratio. Notes: Cox proportional hazards regression model was used for multivariate analysis. Variables were adopted for their prognostic significance by univariate analysis.
Prognostic analysis for leukocyte RTL in HCC patients
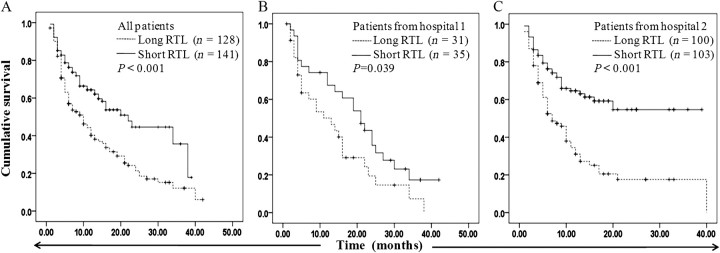
To investigate whether blood RTL had prognostic significance, we divided all the patients into two groups based on the median value (0.60) of their RTLs. Both univariate and multivariate analyses indicated that long leukocyte RTL was significantly associated with poor OS of all HCC patients ( n = 269), with an hazard ratio of 2.16 (95% CI, 1.43–2.98; P < 0.001) and 2.04 (95% CI, 1.46–2.86; P < 0.001), respectively ( Supplementary Table 1 is available at Carcinogenesis Online; Table III ). Furthermore, we performed the same analyses for the 66 patients from Hospital 1 and the 203 patients from Hospital 2 and obtained similar results. Kaplan–Meier analyses showed a significant difference in OS between patients with long and short RTL in the total patients (log rank P < 0.001), the patients from Hospital 1 (log rank P = 0.039) and the patients from Hospital 2 (log rank P < 0.001) ( Figure 1A–C ). Stratified analysis for all patients ( n = 269) by host characteristics showed that the association of RTL with OS was significant in the patients with tumor size ≥ 5 cm ( P < 0.001) but not in the patients with tumor size <5 cm ( P = 0.271) ( Table IV ). Kaplan–Meier analysis showed similar results in the stratified subgroups (data not shown). Due to a small percentage of patients who are female (11.5%, n = 31), those with Child–Pugh score B (8.9%, n = 24) and those with negative HBsAg (11.5%, n = 31), variables sex, Child–Pugh score and HBsAg were not included in the stratified analyses.
Fig. 1.
Kaplan–Meier plots of OS for patients with long and short leukocyte RTL. (A) OS of all patients ( n =269). (B) OS of patients from Hospital 1 ( n =66). (C) OS of patients from Hospital 2 ( n =203).
Table IV.
Overall and stratified analyses on the association between RTL and survival of HCC patients
| Variables | RTL | HR (95%CI) | P value a | Death/total | MST (month) | P value b |
| Age (years) | ||||||
| <54 | Short | 1 | 39/75 | 16 | ||
| Long | 1.96 (1.23–3.12) | 0.004 | 41/57 | 6 | 0.012 | |
| ≥54 | Short | 1 | 17/53 | 34 | ||
| Long | 2.37 (1.37–4.10) | 0.002 | 58/84 | 12 | <0.001 | |
| Liver cirrhosis | ||||||
| Yes | Short | 1 | 40/87 | 16 | ||
| Long | 1.81 (1.20–2.72) | 0.005 | 62/94 | 11 | 0.025 | |
| No | Short | 1 | 16/41 | 34 | ||
| Long | 2.36 (1.28–4.35) | 0.006 | 37/47 | 5 | 0.001 | |
| Tumor size (cm) | ||||||
| <5 | Short | 1 | 12/42 | 34 | ||
| Long | 1.57 (0.73–3.37) | 0.246 | 18/36 | 24 | 0.271 | |
| ≥5 | Short | 1 | 44/86 | 14 | ||
| Long | 2.16 (1.48–3.16) | <0.001 | 81/105 | 6 | < 0.001 | |
| Tumor number | ||||||
| Single | Short | 1 | 23/57 | 23 | ||
| Long | 1.77 (1.06–2.96) | 0.029 | 49/70 | 10 | 0.021 | |
| Multiple | Short | 1 | 33/71 | 20 | ||
| Long | 2.37 (1.49–3.75) | <0.001 | 50/71 | 9 | 0.001 | |
| AFP (μg/l) | ||||||
| <200 | Short | 1 | 16/52 | 25 | ||
| Long | 1.78 (0.97–3.23) | 0.06 | 36/65 | 17 | 0.027 | |
| ≥200 | Short | 1 | 40/76 | 15 | ||
| Long | 2.24 (1.48–3.38) | <0.001 | 63/76 | 6 | < 0.001 | |
| PVT | ||||||
| Yes | Short | 1 | 18/33 | 9 | ||
| Long | 2.95 (1.61–5.43) | <0.001 | 40/44 | 4 | 0.001 | |
| No | Short | 1 | 38/95 | 22 | ||
| Long | 1.57 (1.03–2.37) | 0.035 | 59/97 | 14 | 0.029 | |
| TNM stage | ||||||
| I + II | Short | 1 | 17/57 | 34 | ||
| Long | 1.93 (1.12–2.21) | 0.021 | 29/56 | 19 | 0.038 | |
| III + IV | Short | 1 | 39/71 | 11 | ||
| Long | 2.14 (1.53–3.21) | <0.001 | 70/85 | 6 | 0.001 |
HR: hazard ratio; MST, median survival time. Multivariate Cox proportional hazards regression model was used and confounding factors, such as tumor size, AFP, PVT and Child–Pugh score, were adjusted where appropriate.
P value was determined by multivariate analysis of Cox proportional hazards regression model.
P value was determined by log-rank test.
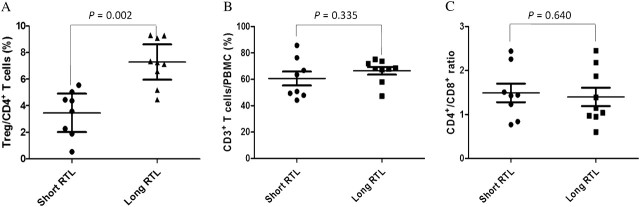
The immunological function of HCC patients with different leukocyte RTLs
To further explore the potential mechanism underlying the leukocyte RTL as a prognostic predictor for TACE-treated HCC patients, we examined the immunological function of HCC patients with different leukocyte RTLs ( Figure 2 ). We divided HCC patients into two groups using the median RTL value (0.70) of 17 samples as a cutoff point. Fluorescence-activated cell sorting analyses showed that the long RTL group ( n = 9) had a significantly increased percentage of CD4 + CD25 + FOXP3 + Treg in CD4 + T cells compared with the short RTL group ( n = 8) ( P = 0.002). However, the percentage of CD3 + T cells in PBMCs and CD4 + /CD8 + T cells ratio had no statistically significant difference between the two groups ( P = 0.335 and P = 0.640, respectively).
Fig. 2.
Immunological function analyses of HCC patients with different leukocyte RTL using flow cytometry. ( A ) Percentage of CD4 + CD25 + FOXP3 + Treg in CD4 + T cells. ( B ) Percentage of CD3 + T cells in PBMCs. ( C ) CD4 + /CD8 + T cells ratio bars indicate mean values ± SD. Each symbol represents one patient. The P values were calculated using an unpaired student’s t test.
Discussion
Various studies have demonstrated that extensive alterations of telomere length might result in genomic abnormalities contributing to cancer development and progression ( 17–20 ). In addition, Svenson et al. ( 21 ) has published a review on the role of tissue telomere length as a prognostic marker in solid tumors, including cancers of lung, breast, prostate, colon and rectum, brain and head and neck. Oh et al. ( 13 ) reported that longer RTL of HCC tissues was associated with worse prognosis. Nonetheless, the majority of these previous studies measured RTL in tissues, which compared with blood are not as easily accessible, especially for non-surgical patients. Furthermore, significant variations exist in the procurement, processing, storage and analyses of tumor tissues. Therefore, there is an urgent need for the development of more reliable easily measurable markers from biospecimens such as blood for the prognostication of malignancies.
In the present study, we measured the RTL in blood from HCC patients treated with TACE-only and examined the associations of RTL with the OS of the patients. Our results clearly indicated a positive correlation between long RTL and poor HCC survival, suggesting that the leukocyte RTL might be used as an independent prognostic biomarker to predict the OS of TACE-treated HCC patients. Consistent with our results, Svenson et al. ( 8 , 9 ) previously reported that longer blood RTL was associated with worse prognosis of breast and renal cancer. The potential mechanism underlying these association remains a task for further investigation. Svenson et al. ( 9 ) have suspected that the downregulation of the immune response in a subset of cancer patients, caused by CD4 + CD25 + FOXP3 + regulatory T cells (Tregs) and/or other immunomodulators, could potentially lead to less telomere attrition due to fewer cell divisions, thus resulting in a reduced antitumoral activity. This theory seems to be biologically plausible and consistent with the observation in our study on the association of long blood RTL with a worse prognosis of cancer patients. Recently, much interest has arisen in the role of a naturally occurring population of regulatory T cells (Tregs) in cancer development. A wealth of evidence obtained using mouse models indicates that Tregs maintain peripheral tolerance to self-antigens and inhibit antitumor immune responses ( 22 , 23 ). Moreover, several groups have reported that the populations of Tregs in peripheral blood and/or tumors were significantly increased and associated with the survival in patients with malignant tumors, including HCC ( 24 ). However, it has not reported whether Tregs play a significant role in the relationship between leukocyte RTL and HCC prognosis.
In this study, we investigated the immunological function of HCC patients with different leukocyte RTLs and found that compared with the HCC patients with short RTL, those with long RTL had a significantly increased percentage of Treg cells, which indicates a decreased ability of immunological activation. Our data suggests a role of Tregs in the modulation of the effect of blood RTL on HCC prognosis. However, as discussed previously, we cannot rule out the possibility that the long telomere length in a subset of patients were due to the elevated levels of diverse telomerase-stimulating factors with combined effects on the hematopoietic system. In future study, more in-depth analyses of blood constituents are needed to identify potential immune-related differences between patients with long versus short blood RTL. In the present study, we did not have data on hepatitis B viral load for most of HCC patients. Therefore, whether hepatitis B viral load contributes to differences in RTL of peripheral blood leukocytes is still needed to be clarified in further study. However, it is well established that high hepatitis B viral load is an independent predictor of poor OS for HCC patients ( 25 ) and is significantly associated with more circulating Treg cells in patients with chronic hepatitis B ( 26 ).
As shown in several earlier studies ( 27–29 ), a significant inverse correlation between RTL and age of the patients was also observed in this study ( Supplementary Figure 1 is available at Carcinogenesis Online). Our data also indicate that RTL in peripheral blood leukocytes might not be associated with clinical features of HCC patients. Similarly, Huang et al. ( 30 ) has reported that neither telomere length nor telomerase activity was correlated with any clinical characteristics of HCC patients. In line with previous studies ( 31 , 32 ), our data showed that tumor size, PVT, Child–Pugh score and AFP were independent prognostic predictors for OS of HCC patients. In addition, stratified analysis by host characteristics showed that the association of RTL with OS was more prominent in patients with advanced diseases, especially in those with large tumor size, which is consistent with a previous report in breast cancer ( 8 ). This finding may reflect the importance of telomere stabilization in tumor progression ( 16 ).
Our study has several strengths. All HCC patients were treated with TACE-only, which greatly reduced the confounding effects of the heterogeneous therapeutic modalities in most cancer clinical outcome studies. In addition, we also have complete demographic and clinical data as well as a low rate of patient loss to follow-up. Moreover, the RTL measurement approach used in this study was based on real-time PCR, which is easy to conduct and yields accurate data. The hypothesis-driven approach targeting a single biomarker of interest eliminated the multiple comparison issue in the majority of current high-throughput screening-based biomarker studies. However, our sample size may not be large enough to detect minimal associations and interactions. Further validations using larger independent populations are warranted.
Overall, our study presents the first epidemiological evidence supporting a role of leukocyte RTL in the prognosis prediction of a Chinese population of HCC patients receiving the mono-treatment of TACE. If validated, RTL may potentially be developed as a simple non-invasive biomarker to help select unresectable HCC patients to receive TACE treatment to achieve the maximum therapeutic benefits. In addition, the same arguments are theoretically valid for more general HCC patients and future studies are warranted to verify whether blood RTL is a helpful prognostic indicator for additional HCC patients receiving other treatments.
Supplementary material
Supplementary Table 1 and Supplementary Data can be found at http://carcin.oxfordjournals.org/ .
Funding
National Natural Science Foundation of China (30872927); National Basic Research Program of China (2009CB521704); National Science and Technology Major Project of China (2009ZX09031-009).
Supplementary Material
Acknowledgments
We thank Professor Chun-Mei Fan and Dr Li-Na Chen (Department of Clinical Immunology, Xijing Hospital, the Fourth Military Medical University, Xi’an, China) for their fluorescence-activated cell sorting analyses.
Conflict of Interest Statement: None declared.
Glossary
Abbreviations
- AFP
alpha-fetoprotein
- CI
confidence interval
- HCC
hepatocellular carcinoma
- OS
overall survival
- PBMC
peripheral blood mononuclear cell
- PVT
portal vein thrombus
- RTL
relative telomere length
- TACE
transarterial chemoembolization
- TNM
tumor-node-metastasis
References
- 1.Bisoffi M, et al. Telomeres: prognostic markers for solid tumors. Int. J. Cancer. 2006;119:2255–2260. doi: 10.1002/ijc.22120. [DOI] [PubMed] [Google Scholar]
- 2.Jang JS, et al. Telomere length and the risk of lung cancer. Cancer Sci. 2008;99:1385–1389. doi: 10.1111/j.1349-7006.2008.00831.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wu X, et al. Telomere dysfunction: a potential cancer predisposition factor. J. Natl Cancer Inst. 2003;95:1211–1218. doi: 10.1093/jnci/djg011. [DOI] [PubMed] [Google Scholar]
- 4.Liu X, et al. Constitutive telomere length and gastric cancer risk: case-control analysis in Chinese Han population. Cancer Sci. 2009;100:1300–1305. doi: 10.1111/j.1349-7006.2009.01169.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Han J, et al. A prospective study of telomere length and the risk of skin cancer. J. Invest. Dermatol. 2009;129:415–421. doi: 10.1038/jid.2008.238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lan Q, et al. A prospective study of telomere length measured by monochrome multiplex quantitative PCR and risk of non-Hodgkin lymphoma. Clin. Cancer Res. 2009;15:7429–7433. doi: 10.1158/1078-0432.CCR-09-0845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gramatges MM, et al. Longer relative telomere length in blood from women with sporadic and familial breast cancer compared with healthy controls. Cancer Epidemiol. Biomarkers Prev. 2010;19:605–613. doi: 10.1158/1055-9965.EPI-09-0896. [DOI] [PubMed] [Google Scholar]
- 8.Svenson U, et al. Breast cancer survival is associated with telomere length in peripheral blood cells. Cancer Res. 2008;68:3618–3623. doi: 10.1158/0008-5472.CAN-07-6497. [DOI] [PubMed] [Google Scholar]
- 9.Svenson U, et al. Telomere length in peripheral blood predicts survival in clear cell renal cell carcinoma. Cancer Res. 2009;69:2896–2901. doi: 10.1158/0008-5472.CAN-08-3513. [DOI] [PubMed] [Google Scholar]
- 10.Tang ZY, et al. A decade’s studies on metastasis of hepatocellular carcinoma. J. Cancer Res. Clin. Oncol. 2004;130:187–196. doi: 10.1007/s00432-003-0511-1. [DOI] [PubMed] [Google Scholar]
- 11.Vogl TJ, et al. Review on transarterial chemoembolization in hepatocellular carcinoma: palliative, combined, neoadjuvant, bridging, and symptomatic indications. Eur. J. Radiol. 2009;72:505–516. doi: 10.1016/j.ejrad.2008.08.007. [DOI] [PubMed] [Google Scholar]
- 12.Yokota T, et al. Telomere length variation and maintenance in hepatocarcinogenesis. Cancer. 2003;98:110–118. doi: 10.1002/cncr.11428. [DOI] [PubMed] [Google Scholar]
- 13.Oh BK, et al. High telomerase activity and long telomeres in advanced hepatocellular carcinomas with poor prognosis. Lab. Invest. 2008;88:144–152. doi: 10.1038/labinvest.3700710. [DOI] [PubMed] [Google Scholar]
- 14.Liu J, et al. Longer leukocyte telomere length predicts increased risk of hepatitis B virus-hyphen;related hepatocellular carcinoma: a case-control analysis. Cancer. 2011;117:4247–4256. doi: 10.1002/cncr.26015. [DOI] [PubMed] [Google Scholar]
- 15.Yokokawa J, et al. Enhanced functionality of CD4+ CD25highFoxP3+ regulatory T cells in the peripheral blood of patients with prostate cancer. Clin. Cancer Res. 2008;14:1032–1040. doi: 10.1158/1078-0432.CCR-07-2056. [DOI] [PubMed] [Google Scholar]
- 16.Deng Y, et al. Telomere dysfunction and tumour suppression: the senescence connection. Nat. Rev. Cancer. 2008;8:450–458. doi: 10.1038/nrc2393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Soler D, et al. Telomere dysfunction drives chromosomal instability in human mammary epithelial cells. Genes Chromosomes Cancer. 2005;44:339–350. doi: 10.1002/gcc.20244. [DOI] [PubMed] [Google Scholar]
- 18.Silva AG, et al. Telomere-centromere-driven genomic instability contributes to karyotype evolution in a mouse model of melanoma. Neoplasia. 2010;12:11–19. doi: 10.1593/neo.91004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Feldser DM, et al. Short telomeres limit tumor progression in vivo by inducing senescence . Cancer Cell. 2007;11:461–469. doi: 10.1016/j.ccr.2007.02.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yoon SY, et al. Telomere length shortening of peripheral blood mononuclear cells in solid-cancer patients undergoing standard-dose chemotherapy might be correlated with good treatment response and neutropenia severity. Acta Haematol. 2007;118:30–37. doi: 10.1159/000101558. [DOI] [PubMed] [Google Scholar]
- 21.Svenson U, et al. Telomere length as a biological marker in malignancy. Biochim. Biophys. Acta. 2009;1792:317–323. doi: 10.1016/j.bbadis.2009.01.017. [DOI] [PubMed] [Google Scholar]
- 22.Liu Z, et al. Tumor regulatory T cells potently abrogate antitumor immunity. J. Immunol. 2009;182:6160–6167. doi: 10.4049/jimmunol.0802664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hori S, et al. Control of regulatory T cell development by the transcription factor Foxp3. Science. 2003;299:1057–1061. [PubMed] [Google Scholar]
- 24.Fu J, et al. Increased regulatory T cells correlate with CD8 T-cell impairment and poor survival in hepatocellular carcinoma patients. Gastroenterology. 2007;132:2328–2339. doi: 10.1053/j.gastro.2007.03.102. [DOI] [PubMed] [Google Scholar]
- 25.Shao YY, et al. Impact of baseline hepatitis B viral DNA levels on survival of patients with advanced hepatocellular carcinoma. Anticancer Res. 2011;31:4007–4011. [PubMed] [Google Scholar]
- 26.Yang GL, et al. Association between CD4+CD25+Foxp3+ regulatory T cells and serum transforming growth factor beta 1 in patients with chronic hepatitis B. Zhonghua Gan Zang Bing Za Zhi. 2009;17:831–834. [PubMed] [Google Scholar]
- 27.Allsopp RC, et al. Telomere length predicts replicative capacity of human fibroblasts. Proc. Natl Acad. Sci. USA. 1992;89:10114–10118. doi: 10.1073/pnas.89.21.10114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Valdes AM, et al. Obesity, cigarette smoking, and telomere length in women. Lancet. 2005;366:662–664. doi: 10.1016/S0140-6736(05)66630-5. [DOI] [PubMed] [Google Scholar]
- 29.Cawthon RM, et al. Association between telomere length in blood and mortality in people aged 60 years or older. Lancet. 2003;361:393–395. doi: 10.1016/S0140-6736(03)12384-7. [DOI] [PubMed] [Google Scholar]
- 30.Huang GT, et al. Telomerase activity and telomere length in human hepatocellular carcinoma. Eur. J. Cancer. 1998;34:1946–1949. doi: 10.1016/s0959-8049(98)00237-8. [DOI] [PubMed] [Google Scholar]
- 31.Shi GM, et al. CD151 modulates expression of matrix metalloproteinase 9 and promotes neoangiogenesis and progression of hepatocellular carcinoma. Hepatology. 2010;52:183–196. doi: 10.1002/hep.23661. [DOI] [PubMed] [Google Scholar]
- 32.Yang XR, et al. High expression levels of putative hepatic stem/progenitor cell biomarkers related to tumour angiogenesis and poor prognosis of hepatocellular carcinoma. Gut. 2010;59:953–962. doi: 10.1136/gut.2008.176271. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.