There are limited data on the efficacy and safety of vascular endothelial growth factor receptor-tyrosine kinase inhibitor (VEGFR-TKI) after programmed cell death (PD-1) inhibition. This study revealed that treatment with VEGFR-TKIs after PD-1 inhibition is safe and retains clinical activity in patients with mRCC.
Keywords: renal cell carcinoma, PD-1 inhibitor, nivolumab, ipilimumab, vascular endothelial growth factor receptor–tyrosine kinase inhibitor
Abstract
Background
Emerging agents blocking the programmed cell death 1 (PD-1) pathway show activity in metastatic clear cell renal cell carcinoma (mRCC). The aim of this study was to evaluate the efficacy and safety of vascular endothelial growth factor (VEGF)/VEGF receptor (VEGFR)–tyrosine kinase inhibitor (TKI) therapy after PD-1 inhibition.
Patients and methods
Patients with mRCC treated with anti-PD-1 antibody (aPD-1) monotherapy or in combination (with VEGFR-TKI or ipilimumab) that subsequently received VEGFR-TKI were retrospectively reviewed. The efficacy end points were objective response rate (ORR) and progression-free survival (PFS) stratified by the type of prior PD-1 regimen. Safety by the type and PD-1 exposure was also evaluated.
Results
Seventy patients were included. Forty-nine patients received prior therapy with immune checkpoint inhibitors (CPIs) alone and 21 had combination therapy of aPD-1 and VEGFR-TKI. Overall, ORR to VEGFR-TKI after PD-1 inhibition was 28% (19/68) and the median PFS was 6.4 months (mo) (4.3–9.5). ORR to VEGFR-TKI after aPD-1 in combination with VEGFR-TKI was lower than that in patients treated with VEGFR-TKI after CPI alone (ORR 10% versus 36%, P = 0.039). In the multivariable analysis, patients treated with prior CPI alone were more likely to achieve an objective response than those treated with aPD-1 in combination with VEGFR-TKI (OR = 5.38; 95% CI 1.12–26.0, P = 0.03). There was a trend toward numerically longer median PFS in the VEGFR-TKI after the CPI alone group, 8.4 mo (3.2–12.4) compared with 5.5 mo (2.9–8.3) for those who had VEGFR-TKI after aPD-1 in combination with VEGFR-TKI (P = 0.15). The most common adverse events (AEs) were asthenia, hypertension, and diarrhea.
Conclusions
The efficacy and safety of VEGFR-TKIs after PD-1 inhibition were demonstrated in this retrospective study. The response rate was lower and the median progression-free survival was shorter in those patients who received prior PD-1 in combination with VEGFR-TKI. PD-1 exposure does not seem to significantly influence the safety of subsequent VEGFR-TKI treatment.
introduction
The therapeutic arsenal for metastatic clear renal cell carcinoma (mRCC) has been expanding considerably over the last decade. Typically two classes of targeted therapies are used sequentially in practice inhibiting either the vascular endothelial growth factor (VEGF)/vascular endothelial growth factor receptor (VEGFR) axis [1–3] or mammalian target of rapamycin (mTOR) [4, 5]. These agents have provided significant clinical benefit for many mRCC patients, but rarely lead to durable responses.
The recent development of checkpoint inhibitor (CPI) including agents targeting the programmed cell death 1 (PD-1) and cytotoxic leukocyte antigen 4 (CTLA-4) pathway has led to disruptive changes in the treatment of solid tumors such as melanoma and lung cancer and will significantly impact the treatment paradigm in kidney cancer. Nivolumab (BMS-936558), a human monoclonal IgG4 antibody, was the first anti-PD-1 antibody (aPD-1) to demonstrate activity and durable responses even in heavily pretreated mRCC patients [6–10]. A recently published phase II trial demonstrated response rates (RRs) of ∼20% across three dose levels studied and encouraging survival data. A phase III trial randomizing patients to either nivolumab or everolimus after having progressed on at least one vascular endothelial growth factor receptor (VEGFR)-tyrosine kinase inhibitor (TKI) has shown a significant survival advantage in favor of nivolumab [11]. Combining nivolumab with other agents such as VEGFR-TKIs or ipilimumab (an anti-CTLA-4 antibody) has also demonstrated encouraging efficacy with a high objective RR in both naive- and pretreated mRCC patients [12, 13].
Therefore, the approval of nivolumab for the treatment of mRCC was recent and is expected to change practice patterns. Limited data are available regarding sequencing PD-1/PD-L1 inhibitors and previously approved targeted therapies. Only one report suggested a sustained benefit of targeted therapies after PD-1/PD-L1 inhibition [14]. With multiple therapeutic options now available to mRCC patients, the question of how PD-1/PD-L1 inhibitors influence safety and outcomes to subsequent VEGFR-TKI therapy remains inadequately characterized.
To address the above question, we hypothesized that prior exposure to the treatment with aPD-1 monotherapy (nivolumab) or in combination (with VEGFR-TKI or ipilimumab) may modify the toxicity profile and clinical activity of subsequent VEGFR-TKI therapy in mRCC patients. Although two classes of targeted therapies have been FDA-approved for the treatment of such patients, we focused on VEGFR-TKIs for the purpose of this study. To assess the safety and efficacy of VEGFR-TKIs in the post-PD-1/PD-L1 inhibition setting, we carried out a retrospective review of consecutive mRCC patients from multiple academic centers who had received a prior PD-1 pathway inhibitor and were subsequently treated with a VEGFR-TKI.
materials and methods
This was a retrospective, multi-institutional study that included patients with mRCC enrolled in one of the three prospective trials (Clinicaltrials.gov identifiers NCT01668784, NCT01472081, and NCT01472081) evaluating either single-agent aPD-1 or aPD-1 in combination with a CTLA-4 inhibitor (ipilimumab) or VEGFR-TKI; all patients included in this analysis were subsequently treated with a VEGFR-TKI as a part of standard management. Patients were required to have disease progression by investigator assessment. FDA-approved TKIs such as sunitinib, pazopanib, axitinib, or sorafenib were used at the discretion of the treating provider. Patients were grouped into patients who received combination therapy of aPD-1 and VEGFR-TKI and those who received CPI alone (either aPD-1 monotherapy or aPD-1 in combination with ipilimumab). Institutional review board approval was obtained before data collection at each institution
clinical variables
Electronic medical records were reviewed for the following characteristics: patient gender, histology (pure clear cell carcinoma versus clear cell component), Fuhrman/nuclear grade, presence of sarcomatoid features (yes versus no), and time from diagnosis or metastatic RCC to initiation of first systemic treatment (<1 year or not). Details on treatments before PD-1 inhibitor use were categorized as prior HD-IL2 treatment, antiangiogenic (sunitinib, pazopanib, sorafenib, axitinib, tivozanib, or bevacizumab) and mTOR inhibitor treatment (temsirolimus or everolimus). The objective RR (ORR) and length of time the patient received PD-1-preceding antiangiogenic or mTOR inhibitor therapy (defined as time from the start of therapy until disease progression, discontinued because of unacceptable toxicity or treatment change) were also documented. The start of any new systemic therapy, including an antiangiogenic agent, mTOR inhibitor, or an investigational therapy, was considered a treatment change.
Baseline Karnofsky performance status (PS; <80% versus ≥80%), LDH level, hemoglobin level, corrected calcium level, neutrophil and platelet count, presence of brain metastases, and extent of the disease (1 versus >1 metastasis sites) were collected at the time of VEGFR-TKI initiation after aPD-1 therapy. Patients were categorized as: (i) favorable risk; (ii) intermediate risk; and (iii) poor risk according to both the Memorial Sloan Kettering Cancer Center (MSKCC) (three prognostic factor model: Karnofsky PS, hemoglobin level, and corrected serum calcium) [15] and The International Metastatic Renal Cell Carcinoma Database Consortium (IMDC) risk criteria [16].
clinical outcomes and safety
Response rate (RR) on VEGFR-TKI was assessed using Response Evaluation Criteria in Solid Tumors RECIST 1.1 [17]. The baseline scan was the scan obtained just before the start of chemotherapy. Patients were grouped into four categories according to their best response during treatment: complete response (CR), partial response (PR), stable disease (SD), and progressive disease (PD). PD was also considered if the patient had clinical progression as determined by the treating physician at the time of last follow-up. ORR included the proportion of patients in whom a CR or PR was observed. Because patients were treated off protocol, response confirmation was not required to meet criteria for response.
Progression-free survival (PFS) was calculated from the date of subsequent VEGFR-TKI initiation to investigator-assessed clinical progression or radiographic progression (by RECIST) or death from any cause (whichever occurred first). Those who were alive with no disease progression were censored at the date of last visit. Overall survival (OS) was defined as the time from initiation of VEGFR-TKI therapy to death from any cause. Those who were still alive at the time of analysis were censored at the date of last evaluation.
Safety was assessed throughout the VEGFR-TKI after PD-1 inhibition, and the severity of adverse events (AEs) was evaluated using the National Cancer Institute Common Toxicity Criteria version 4.0. AEs leading to VEGR-TKI discontinuations, treatment delays, specific intervention treatment, or hospital admissions were collected by patient chart review at each center and graded accordingly (grades 2–4). The grading of laboratory abnormalities was based on local laboratory cutoffs. Safety data for the entire cohort were summarized descriptively. Patients were also stratified by prior aPD1 exposure (<median duration of PD-1 inhibition and ≥median duration of PD-1 inhibition) to determine whether the length of exposure to aPD-1 has an impact on safety with VEGFR-TKI after PD-1 inhibition.
statistical analysis
Numerical variables were summarized with the sample median and range. Categorical variables were summarized with frequencies and percentages. Clinical outcomes of interest included ORR, PFS, and OS. Differences in ORR between cohorts were compared using Fisher's exact test and the trend in best overall response was further evaluated using the exact Cochran–Armitage test. PFS and OS were described using the Kaplan–Meier method and differences in survival distributions between groups were determined via the long-rank test or the Fleming–Harrington test that gives relatively more weight on differences between survival functions in the later time points. Exploratory analyses were carried out to identify variables associated with the above clinical outcomes with VEGFR-TKI therapy after PD-1 inhibition. Univariate and multivariate logistic regression analyses were carried out to evaluate the association of baseline clinical and pre-PD-1 inhibition treatment features with tumor response (CR or PR) to subsequent VEGFR-TKI. Univariate and multivariate Cox proportional hazard models were also used to investigate the influence of baseline clinical and pre-PD-1 inhibition treatment features with PFS and OS. Variables found to be statistically significant on univariate analyses as well as those deemed clinically important were included in the multivariable analyses. No adjustment for multiple testing was made in these exploratory analyses. All tests were two-sided and considered statistical significant at P < 0.05. Statistical analysis was carried out using SAS (v 9.3.) and R statistical software (v 3.1.0).
results
patient's characteristics
Between December 2011 and December 2014, 70 patients with mRCC received a VEGFR-TKI after PD-1 inhibition. Two patients were excluded from the RR analysis secondary to not being evaluable for tumor response. This resulted in a final cohort of 68 patients who received a VEGFR-TKI after PD-1 inhibition for metastatic RCC. Accurate data on time from the PD-1 inhibition to initiation of VEGFR-TKI were only available in 64 patients. The median follow-up time since the initiation of VEGFR-TKI after aPD-1 therapy was 7.8 months (range 0.2–38.9). Forty-nine patients received prior therapy with aPD-1 monotherapy or aPD-1 in combination with ipilimumab, and 21 had combination therapy of aPD-1 and VEGFR-TKI. Two-thirds of the patients were men and most of the patients had previously undergone a nephrectomy. Patient's characteristics are summarized in Table 1.
Table 1.
Baseline patients' characteristics (N = 70)
| Characteristics | n (%) |
|---|---|
| Demographics | |
| Gender | |
| Male | 45 (64.3) |
| Female | 25 (35.7) |
| Histology | |
| Clear cell carcinoma | 67 (95.7) |
| Clear cell carcinoma component | 3 (4.3) |
| Fuhrman grade (primary kidney tumor) | |
| 2 | 9 (12.9) |
| 3 | 33 (47.1) |
| 4 | 18 (25.7) |
| Unknown | 10 (14.3) |
| Sarcomatoid features | |
| Present | 10 (14.3) |
| Absent | 28 (40.0) |
| Unknown | 32 (45.7) |
| Prior nephrectomy | |
| Yes | 69 (98.6) |
| No | 1 (1.4) |
| Prior targeted therapies | |
| High dose—interleukin 2 | |
| Yes | 23 (32.9) |
| No | 47 (67.1) |
| Number of prior antiangiogenic therapiesa | |
| 0 | 18 (25.7) |
| 1 | 39 (55.7) |
| 2–3 | 13 (18.6) |
| Prior mTOR inhibitor | |
| Yes | 7 (10.0) |
| No | 63 (90.0) |
| Type of PD-1 inhibitor regimen | |
| PD-1 inhibitor monotherapy | 32 (45.7) |
| aPD-1 in combination with VEGFR-TKI | 21 (30.0) |
| aPD-1 in combination CTLA-4 inhibitor | 17 (24.3) |
aSunitinib, pazopanib, sorafenib, axitinib, bevacizumab, tivozanib, ziv-aflibercept; combination of sunitinib–bevacizumab.
mTOR: mammalian target of rapamycin; PD-1: programmed cell death 1; aPD-1: anti-PD-1 antibody; VEGFR-TKI: vascular endothelial growth factor receptor–tyrosine kinase inhibitors; CTLA: cytotoxic leukocyte antigen 4.
The median age before the initiation of VEGFR-TKI was 59 years (range 27–82). All patients had more than one metastatic site and 14 (20%) patients had brain metastasis. By the MSKCC prognostic risk group criteria [15], 41.4% of patients had intermediate risk, 30% had good risk, and 27.1% had poor risk.
Thirteen of these patients (18.6%) received 2–3 lines and 39 (55.7%) received one line of antiangiogenic therapies before aPD-1 therapy. Eighteen (25.7%) were systemic treatment-naïve at the time of the aPD-1 therapy initiation. The median length of first-line and second-line antiangiogenic therapy was 9.0 months (mo; range 2–69) and 6.5 mo (range 2–56), respectively. Axitinib was the most common choice for VEGFR-TKI after PD-1 inhibition (67.1%) followed by sunitinib (15.7%), pazopanib (14.3%), and less frequently sorafenib (2.9%). The median time from the end of treatment with aPD-1 to subsequent VEGFR-TKI was 0.8 mo (range 0–16.4; Table 2). Three patients had intrinsic resistance to first-line VEGFR-TKI and were subsequently treated with single-agent nivolumab. Fifty patients (66.6%) progressed by the time of the analysis.
Table 2.
Patients' characteristics before VEGFR-TKI therapy after PD-1 inhibition (N = 70)
| Characteristics | n (%) |
|---|---|
| Age (years) | |
| Median (range) | 59 (27–82) |
| Number of metastatic sites | |
| ≥2 | 70 (100) |
| Presence of brain metastasis | |
| Yes | 14 (20.0) |
| No | 56 (80.0) |
| Heng's prognostic group | |
| Good | 11 (15.7) |
| Intermediate | 38 (54.3) |
| Poor | 20 (28.6) |
| Unknown | 1 (1.4) |
| MKSCC prognostic group | |
| Good | 21 (30.0) |
| Intermediate | 29 (41.4) |
| Poor | 19 (27.1) |
| Unknown | 1 (1.4) |
| Type of VEGFR-TKI | |
| Axitinib | 47 (67.1) |
| Pazopanib | 10 (14.3) |
| Sunitinib | 11 (15.7) |
| Sorafenib | 2 (2.9) |
| Time from the end of PD-1 therapy to subsequent VEGF/VEGFR-TKI therapy (mon)a | |
| Median (range) | 0.8 (0–16.7) |
aSunitinib, pazopanib, sorafenib, axitinib, bevacizumab, tivozanib, ziv-aflibercept; combination of sunitinib–bevacizumab.
PD-1: programmed cell death 1; aPD-1: anti-PD-1 antibody; VEGFR-TKI: vascular endothelial growth factor receptor–tyrosine kinase inhibitors; mon: Months.
efficacy outcomes of VEGFR-TKI after PD-1 inhibition
A total of 68 patients were evaluable for response. ORR to VEGFR-TKI after PD-1 inhibition was 28% (19/68) for the entire population. ORR to VEGFR-TKI after PD-1 inhibition was significantly lower in patients previously treated with aPD-1 in combination with VEGFR-TKI when compared with those treated with VEGFR-TKI after CPI alone (ORR 10% versus 36%, P = 0.039, Fisher's exact test). There appeared to be a clear trend in the objective tumor response according to pre-PD-1 inhibition treatment features (P = 0.009, Cochran–Armitage test). SD as the best response was noted in ∼40% of patients in both groups (Table 3).
Table 3.
Objective response by prior aPD-1 regimen
| Objective response | Immune checkpoint inhibitors (n = 47) | aPD-1 combined with VEGFR-TKI (n = 21) | P-value |
|---|---|---|---|
| ORR | 17 (36.2) | 2 (10.0) | 0.039 (Fisher's exact test) |
| PR | 17 (36.2) | 2 (9.5) | 0.009 (exact Cochran–Armitage trend test) |
| SD | 20 (42.6) | 9 (42.9) | |
| PD | 10 (21.3) | 10 (47.6) |
aPD-1: antibody anti-PD-1; immune checkpoint inhibitors refer to aPD-1 monotherapy or in combination with a CTLA-4 inhibitor; VEGFR-TKI refers to axitinib, sunitinib, pazopanib, and sorafenib; ORR: objective response rate; PR: partial response; SD: stable disease; PD: progressive disease.
The univariate logistic regression analysis for ORR suggested that patients who had prior CPI alone were 5.38 times more likely to achieve an objective response while on VEGFR-TKI than those who had prior aPD-1 in combination with VEGFR-TKI (OR = 5.38; 95% CI 1.12–26.0, P = 0.03). Longer interval from the end of aPD-1 therapy to initiation of subsequent VEGFR-TKI appeared to be associated with smaller odds of achieving response (OR 0.63; 95% CI 0.36–1.01; P = 0.05). Detailed results of the univariate analysis are listed in supplementary Table S1, available at Annals of Oncology online. Differences in baseline clinical and pretreatment characteristics were controlled for by constructing a multivariable logistic regression model. In this analysis, the association between the type of PD-1 inhibition regimen and interval from aPD-1 therapy to the initiation of subsequent VEGFR-TKI persisted with respect to ORR (Table 4). The interaction effect between the time from the end of aPD-1 therapy to the initiation of subsequent VEGFR-TKI and the type of PD-1 inhibitor regimen was not statistically significant (P = 0.45), suggesting that the association of this time interval with objective response did not differ between patients who received CPI alone and those who received aPD-1 in combination with VEGFR-TKI.
Table 4.
Multivariable analysis of association with clinical outcomes to VEGFR-TKI after PD-1
| Variable | Objective response |
Progression-free survival |
||
|---|---|---|---|---|
| OR (95% CI) | P-value | RR (95% CI) | P-value | |
| IL-2 | ||||
| Yes versus no | 0.51 (0.09–2.79) | 0.437 | 1.10 (0.55–2.21) | 0.781 |
| Number of prior antiangiogenic therapies | ||||
| ≥1 versus 0 | 0.44 (0.11–1.70) | 0.233 | 1.19 (0.48–0.95) | 0.714 |
| MSKCC's criteria | ||||
| Good versus poor | 1.69 (0.28–10.1) | 0.391 | 0.71 (0.30–1.68) | 0.437 |
| Intermediate versus poor | 0.73 (0.15–3.63) | 0.419 | 0.62 (0.31–1.26) | 0.191 |
| Good versus intermediate | 2.31 (0.42–12.7) | 0.381 | 1.14 (0.49–2.64) | 0.757 |
| Time from the end of PD-1 therapy to subsequent VEGFR-TKI therapy | 0.50 (0.27–0.94) | 0.030 | 0.95 (0.85–1.06) | 0.365 |
| Type of PD-1 inhibitor regimen | ||||
| Immune checkpoint inhibitors alone versus PD-1 combined with VEGFR-TKI therapy | 5.83 (1.04–32.7) | 0.045 | 0.62 (0.29–1.32) | 0.218 |
Values in bold are statistically significant.
PD-1: programmed cell death 1; VEGFR-TKI: vascular endothelial growth factor receptor–tyrosine kinase inhibitors.
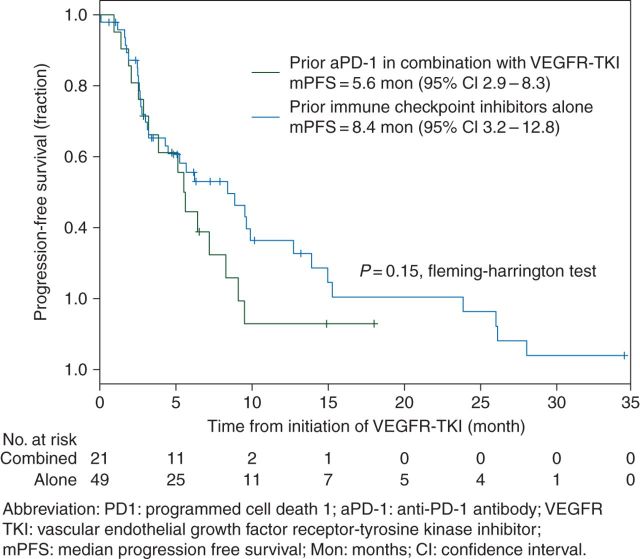
The median PFS with VEGFR-TKI after PD-1 inhibition for the entire cohort was 6.4 mo (4.3–9.5). Stratified analysis by the type of PD-1 inhibition regimen showed a trend toward numerically longer median PFS in the VEGFR-TKI after the CPI alone group, 8.4 mo (3.2–12.4) compared with 5.5 mo (2.9–8.3) for those who had a prior VEGFR-TKI after aPD-1 in combination with the VEGFR-TKI group, although the PFS distributions between these two groups were not statistically significant (P = 0.15, Fleming–Harrington test, Figure 1).
Figure 1.
Progression-free survival curve by the type of PD-1 treatment. PD1: programmed cell death; aPD1: anti-PD-1 antibody; VEGFR-TKI: vascular endothelial growth factor receptor–tyrosine kinase inhibitor; mPFS: median progression-free survival; Mon: months; CI: confidence interval.
The univariate Cox proportional hazard model for PFS suggested that patients who had prior CPI alone had 32% less risk of developing disease progression or death than those who had prior aPD-1 in combination with VEGFR-TKI, albeit not statistically significant (HR 0.68, 95% CI 0.37–1.26, P = 0.22). Detailed results are displayed in supplementary Table S2, available at Annals of Oncology online. The multivariable Cox proportional hazard analysis suggested a persistent result that patients who received prior therapy with CPI alone had a trend toward less risk of developing disease progression or death than those who had prior aPD-1 in combination with VEGFR-TKI (HR 0.62; 95% CI 0.29–1.32, P = 0.21). No association was evident between the interval from the end of treatment with aPD-1 to the initiation of VEGFR-TKI and progression on VEGFR-TKI (HR 0.95, 95% CI 0.85–1.06, P = 0.376; Table 4).
The median OS was 16.9 mo (10.7–25.6) in the entire population. The median OS was 16.3 mo (10.7–25.6) in the VEGFR-TKI after CPI alone and 24.9 mo (6.3–NA) in the VEGFR-TKI after aPD-1 in combination with the VEGFR-TKI group. We then carried out a multivariate Cox regression analysis in a subgroup of patients who received VEGFR-TKI after immune-CPI alone including prior HD-IL2 exposure, number of antiangiogenic therapies, MSKCC's criteria, and interval from the end of treatment with PD-1 inhibition to initiation of VEGFR-TKI to determine whether potential clinical factors are associated with clinical outcomes in this particular subgroup of patients. Longer interval from aPD-1 therapy to initiation of subsequent VEGFR-TKI also resulted in lower ORR (ORR 0.54; 95% CI 0.30–0.98, P = 0.04). Detailed univariate and multivariate analysis are shown in supplementary Table S3, available at Annals of Oncology online.
In this study, 17 patients received VEGFR-TKI after aPD-1 in combination with a CTLA-4 inhibitor. In this subset of patients (16 evaluable for response), 5 patients achieved PRs, yielding an ORR of 31.3%. Seven (43.8%) additional patients had SD. The median PFS was 8.9 months (95% CI 2.8–NA).
safety
Any AE occurred in 90% of the patients. Overall, the most common AEs (all grades) in the patients were asthenia (72%) and hypertension (39%). Hypothyroidism was reported in ∼24% of the cases. Table 5 summarizes AEs of the entire population. One patient treated with pazopanib experienced grade 3 hepatotoxicity. This patients started pazopanib 16 days after single-agent aPD1 discontinuation. Baseline AST and ALT before pazopanib treatment were within normal limits.
Table 5.
Toxicity profile
| Adverse events | All grades | Grades 3–4 |
|---|---|---|
| Asthenia | 49 (72%) | 7 (10%) |
| Hypertension | 27 (39%) | 2 (3%) |
| Hypothyroidism | 17 (25%) | 1 (1.5%) |
| Diarrhea | 17 (25%) | 0 |
| Dysphonia | 16 (23.5%) | 0 |
| Anemia | 13 (19%) | 2 (3%) |
| Thrombocytopenia | 11 (16%) | 2 (3%) |
| Hand–Foot Syndrome | 9 (13%) | 1 (1.5%) |
| Leukopenia | 8 (12%) | 2 (3%) |
| Hepatotoxicitya | 8 (12%) | 1 (1.5%) |
| Increased creatinine | 8 (12%) | 0 |
| Nausea | 7 (10%) | 1 (1.5%) |
| Proteinuriab | 3 (6.4%) | 0 |
| Colitis | 4 (6%) | 2 (3%) |
| Mucositis | 4 (6%) | 1 (1.5%) |
| Weight loss | 4 (6%) | 0 |
| Dysgeusia | 3 (4%) | 0 |
| Arthralgia | 2 (3%) | 1 (1.5%) |
| Rash | 2 (3%) | 0 |
aAST and ALT elevations.
bOnly available data in 47 patients.
Twenty-three percent of patients developed grades 1–2 dysphonia and 12% had hand–foot syndrome, which is consistent with AEs induced by axitinib (most commonly used VEGFR-TKI). Safety by PD-1 exposure was also evaluated (<median duration of PD-1 inhibition versus ≥median duration of PD-1 inhibition) and is displayed in supplementary Table S4, available at Annals of Oncology online.
discussion
Agents blocking the PD-1/PD-L1 pathway represent a new treatment option for mRCC and other cancers. The result from a randomized phase III trial (CHECKMATE 025) positions nivolumab as the first PD1 inhibitor to be approved for mRCC in patients having progressed on one to two prior lines of therapy and wide adoption of this agent is anticipated. However, with a median PFS of 4–5 months, the majority of these patients will be treated with nivolumab only temporarily and subsequent therapies will be required. In this retrospective study, we demonstrated that treatment with VEGFR-TKI has anti-tumor activity and can be done safely after PD-1 inhibition. Overall, the ORR to VEGFR-TKI after PD-1 inhibition was 28% and the median PFS was of 6.4 mo (4.3–9.5).
Consistent with our results, another retrospective analysis of targeted therapies (including VEGFR-TKI and mTOR inhibitors) after PD-1 or PD-L1 inhibition demonstrated anticancer activity in patients with metastatic RCC after PD1/PD-L1 inhibition with a median time to treatment failure of 6.9 mo for VEGFR-TKIs (n = 43) and 5.9 mo for mTOR inhibitors (n = 13) [13]. Our analysis focused only on VEGFR-TKI and the decision to use this type of agent after PD-1 inhibition has been made at the discretion of the treating physician. The adoption of this strategy was likely influenced by some investigators' concerns about the immunosuppressive effect of mTOR inhibitors and individual patient characteristics. Taken together, these studies suggest that metastatic RCC patients derive benefit from VEGFR-TKI after PD-1 or PD-L1 inhibition and immune-related AEs were rare.
In the present analysis, stratification based on prior PD-1 inhibition regimen showed that patients who received prior CPI alone more than tripled the ORR compared with those who received aPD-1 in combination with VEGFR-TKI. However, the improvement in RR did not translate in a statistically significant difference in PFS (8.4 versus 5.6 mo; P = 0.15) in our cohort. Although these subsets were very small, the differences in ORR and median PFS are consistent with existing data showing a reduced clinical effect of VEGF-targeting agents after prior exposure to VEGF-targeting agents [3]. If this holds true, alternative treatment strategies or optimization of VEGFR-TKI therapy after the combination of aPD1 and VEGFR-TKI would ultimately be required to promote higher RRs and longer disease control.
Interestingly, in this analysis, we also observed that longer interval from the end of treatment with aPD-1 to initiation of VEGFR-TKI had adverse impact on ORR to VEGFR-TKI in both patients who received prior CPI alone and in the entire population. This may imply a potentially overlapping activity between residual PD-1 inhibition and VEGFR-TKI activity that might have a positive effect on subsequent VEGFR-TKI efficacy. On the other hand, it should be noted in our cohort both patients who discontinued aPD1 due to side-effects or disease progression were included in the analysis. This might have biased and falsely improved the ORR with a subsequent VEGFR-TKI in those patients who stopped aPD1 because of safety reasons and potentially an early response to aPD1 or leading to a synergetic effect of aPD1 and VEGFR-TKI. Therefore, this potential relationship needs to be further assessed in a larger cohort. Importantly, those patients that were treated with first-line CPI alone followed by a VEGFR-TKI achieved an ORR and median PFS (8.9 months) and these outcomes were compared with outcomes with first-line VEGFR-TKI treatment in patients with mRCC.
On the basis of the preliminary data on the safety of the combination of pembrolizumab and pazopanib in advance RCC causing significant hepatotoxicity, balance between efficacy and safety will play a pivotal role in the selection of the optimal aPD1 and VEGFR-TKI combination for clinical practice [18]. In our study, the most common VEGF TKI used was axitinib. Although these data should be interpreted with caution, the finding that PD-1 exposure does not seems to influence the toxicity profile of subsequent VEGFR-TKI is important and should serve to reassure oncologists and patients about their subsequent use. Owing to the data collection process, patients with mild AEs during VEGFR-TKI therapy may be underestimated in this study; however, those patients who had moderate–severe, clinically significant, AEs resulting in an intervention were apparent and captured. In summary, our results suggest that currently FDA-approved VEGFR-TKIs are safe agents after PD-1 inhibition. Owing to the small sample size, however, our data do not assist in the selection of a particular agent for subsequent VEFGR-TKI after PD-1 inhibition based on safety.
Limitations of this study include its relatively small sample size and the inherent bias in retrospective analyses. Importantly, we included only patients who were able to receive therapy after PD-1 inhibition, thus excluding patients with rapidly PD. The comparison of clinical outcomes between CPI alone-treated and aPD-1 in combination with VEGFR-TKI-treated patient was also confounded by the fact that treatment selection was not randomly assigned and that baseline patient characteristics (including number and types of prior therapies received and patients with intrinsic resistance to VEGFR-TKIs) were different in the two cohorts. In particular, the OS analysis most likely was influenced by differences in line of therapy since the Checkmate 016 Ipilimumab and Nivolumab arm (NCT01472081) included only patients treated with VEGFR-TKIs in the adjuvant or neoadjuvant setting or patients treated with cytokines for metastatic disease, therefore demonstrating the TKI-related OS benefit for essentially VEGFR-TKI-naïve patients. In addition, the lack of adjustment for subsequent treatments may have contaminated the OS comparison between arms.
Nevertheless, this represents the largest reported multi-institutional sample of patients with metastatic RCC treated with VEGFR-TKIs after PD-1 inhibition. Thus, our results can be used as a benchmark for the design of future clinical trials in the post-PD-1 inhibition setting.
conclusion
Patients with mRCC who discontinued treatment with aPD-1 either in monotherapy or in combination with a CTLA-4 inhibitor or VEGFR-TKI can benefit from subsequent treatment with VEGFR-TKIs without a noted increase in toxicity in this cohort. Patients who received VEGFR-TKI after CPI alone were more likely to respond and had a trend toward longer PFS compared with those who received prior aPD-1 in combination with VEGFR-TKI. Our findings require further validation in a larger cohort and prospective evaluation of treatment strategies in the post-PD1 setting will be ultimately required.
funding
DMG has received research funding from Pfizer (grant number 11103561). ERP has received research funding from Pfizer, Bristol-Myers Squibb, Acceleron, GlaxoSmithKline, and AstraZeneca (no grant numbers applied). MHV has research funding from Pfizer and Bristol-Myers Squibb (no grant numbers applied). HJH has received clinical trial funding from Bristol-Myers Squibb (grant number DOD CA140917).
disclosure
HJH certifies that all conflicts of interest, including specific financial interests and relationships and affiliations relevant to the subject matter or materials discussed in the manuscript (e.g. employment/affiliation, grants or funding, consultancies, honoraria, stock ownership or options, expert testimony, royalties, or patents filed, received, or pending), are the following: HJH has clinical trial research funding from BMS, Exelixis, Pfizer, and Newlink and has been a consultant for BMS and Exelixis. DMG has been paid advisory board/honoraria from Pfizer, Novartis, and Prometheus. ERP has been paid advisory board/honoraria from Novartis, Genentech, Pfizer, and Bristol-Myers Squibb. MHV has been paid advisory board/honoraria from Bayer, Novartis, GSK, and Calithera. All remaining authors have declared no conflicts of interest.
Supplementary Material
references
- 1. Motzer RJ, McCann L, Deen K. Pazopanib versus sunitinib in renal cancer. N Engl J Med 2013; 369: 1970. [DOI] [PubMed] [Google Scholar]
- 2. Motzer RJ, Escudier B, Tomczak P et al. . Axitinib versus sorafenib as second-line treatment for advanced renal cell carcinoma: overall survival analysis and updated results from a randomised phase 3 trial. Lancet Oncol 2013; 14: 552–562. [DOI] [PubMed] [Google Scholar]
- 3. Rini BI, Escudier B, Tomczak P et al. . Comparative effectiveness of axitinib versus sorafenib in advanced renal cell carcinoma (AXIS): a randomised phase 3 trial. Lancet 2011; 378: 1931–1939. [DOI] [PubMed] [Google Scholar]
- 4. Hudes G, Carducci M, Tomczak P et al. . Temsirolimus, interferon alfa, or both for advanced renal-cell carcinoma. N Engl J Med 2007; 356: 2271–2281. [DOI] [PubMed] [Google Scholar]
- 5. Motzer RJ, Escudier B, Oudard S et al. . Efficacy of everolimus in advanced renal cell carcinoma: a double-blind, randomised, placebo-controlled phase III trial. Lancet 2008; 372: 449–456. [DOI] [PubMed] [Google Scholar]
- 6. Brahmer JR, Drake CG, Wollner I et al. . Phase I study of single-agent anti-programmed death-1 (MDX-1106) in refractory solid tumors: safety, clinical activity, pharmacodynamics, and immunologic correlates. J Clin Oncol 2010; 28: 3167–3175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Topalian SL, Hodi FS, Brahmer JR et al. . Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med 2012; 366: 2443–2454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Motzer RJ, Rini BI, McDermott DF et al. . Nivolumab for metastatic renal cell carcinoma: results of a randomized phase II trial. J Clin Oncol 2014; 33: 1430–1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Charles G, Drake DFM, Sznol M et al. . Survival, safety, and response duration results of nivolumab (anti-PD-1; BMS-936558; ONO-4538) in a phase I trial in patients with previously treated metastatic renal cell carcinoma (mRCC): long-term patient follow-up. J Clin Oncol 2013; 31(suppl; abstr 4514) 2013. [Google Scholar]
- 10. McDermott DF, Drake CG, Sznol M et al. . Survival, durable response, and long-term safety in patients with previously treated advanced renal cell carcinoma receiving nivolumab. J Clin Oncol 2015; 33: 2013–2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Motzer RJ, Escudier B, McDermott DF et al. . Nivolumab versus everolimus in advanced renal-cell carcinoma. N Engl J Med 2015; 373: 1803–1813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hans J, Hammers ERP, Infante JR et al. . Phase I study of nivolumab in combination with ipilimumab in metastatic renal cell carcinoma (mRCC). J Clin Oncol 2014; 32: 5s.(suppl; abstr 4504) 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Asim Amin ERP, Infante JR, Ernstoff MS et al. . Nivolumab (anti-PD-1; BMS-936558, ONO-4538) in combination with sunitinib or pazopanib in patients (pts) with metastatic renal cell carcinoma (mRCC). J Clin Oncol 2014; 32: 5s.(suppl; abstr 5010) 2014. [Google Scholar]
- 14. Albiges L, Fay AP, Xie W et al. . Efficacy of targeted therapies after PD-1/PD-L1 blockade in metastatic renal cell carcinoma. Eur J Cancer 2015; 51: 2580–2586. [DOI] [PubMed] [Google Scholar]
- 15. Motzer RJ, Bacik J, Schwartz LH et al. . Prognostic factors for survival in previously treated patients with metastatic renal cell carcinoma. J Clin Oncol 2004; 22: 454–463. [DOI] [PubMed] [Google Scholar]
- 16. Heng DY, Xie W, Regan MM et al. . Prognostic factors for overall survival in patients with metastatic renal cell carcinoma treated with vascular endothelial growth factor-targeted agents: results from a large, multicenter study. J Clin Oncol 2009; 27: 5794–5799. [DOI] [PubMed] [Google Scholar]
- 17. Eisenhauer EA, Therasse P, Bogaerts J et al. . New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 2009; 45: 228–247. [DOI] [PubMed] [Google Scholar]
- 18. McDermott JRI DF, Chowdhury S, Voss MH et al. . A phase I/II study to assess the safety and efficacy of pazopanib (paz) and pembrolizumab (pembro) in patients (pts) with advanced renal cell carcinoma (aRCC). ESMO 2015; abstract P113. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.