Abstract
Mucormycosis is a devastating invasive fungal disease whose incidence has increased during the past decade. Mucormycosis now represents a major threat in transplant recipients, accounting for 2% and 8% of invasive fungal infections in recent cohorts of solid-organ and allogeneic stem-cell transplant recipients, respectively. Mucormycosis most often occurs late, >3 months after transplantation, although cases occurring early have been observed, especially among liver transplant recipients and in cases of graft-transmitted infection. Recent guidelines have emphasized the direct examination of the involved fluid or tissue and culture from a sterile site as the most appropriate diagnostic strategy and the use of lipid formulations of amphotericin B and major surgery when feasible as the most appropriate first-line therapeutic strategy for mucormycosis in organ and stem cell transplant recipients.
Mucormycosis refers to a group of potentially lethal opportunistic mycoses that occur in immunocompromised or diabetic patients and are caused by Mucorales, ubiquitous filamentous fungi with broad, thin-walled, sparsely septate, ribbon-like hyphae. Pathogenic Mucorales include approximately 10 genera and 20 species, the most common of which are Rhizopus species, Lichtheimia species (former Absidia species), Rhizomucor species, Mucor species, and Cunninghamella species. In healthy persons, innate immunity is sufficient to prevent infection with these fungi, except in cases of massive contamination due to traumatic inoculation of contaminated soil. During infection, the hyphae invade blood vessels, causing tissue infarction and necrosis.
In the past 2 decades, mucormycosis has emerged as an important invasive fungal infection in solid-organ transplant (SOT) and hematopoietic stem-cell transplant (HSCT) recipients, in whom it often has an aggressive clinical course and substantial rates of death [1–3]. Although several risk factors may be found in these 2 populations, the potent T cell–depleting drugs and/or antibodies used for immunosuppression in transplant recipients are responsible for the groups’ high risk of invasive fungal infection [4, 5].
Most studies dealing with mucormycosis in transplant recipients have been descriptive and retrospective, and many of these have been single-institution studies. Results are therefore difficult to compare because of the lack of uniform diagnostic criteria, the potential geographical variation in exposures, and variation in transplant procedures, including the potential influence of the volume of transplant procedures on fungal incidence [6]. In addition, the lack of good recent autopsy data from transplant recipients makes it difficult to appropriately document epidemiological trends of mucormycosis in this population. However, some recent large epidemiological and risk factor studies have helped to better clarify the current picture of mucormycosis in transplant recipients.
The aim of the present article is to provide a review of the most recent data regarding the epidemiology, risk factors, clinical presentation, therapeutic recommendations, and outcomes of mucormycosis in SOT and HSCT recipients.
EPIDEMIOLOGY
Characteristics of SOT
Recently published estimated incidences of mucormycosis in SOT recipients have ranged from 0.4% to 16.0%, depending on the procedure and the geographical area [4, 7–10]. Older studies found overall incidences of 0.2%–2%, 0%–2%, 0%–3%, and 0%–3% in kidney, liver, heart, and lung transplant recipients, respectively [11–17]. The recent prospective multicenter TRANSNET study in the United States found that the 12-month cumulative incidence of mucormycosis was 0.07% in SOT recipients, with mucormycosis accounting for 2% of invasive fungal infections [18] (Figure 1). Almyroudis et al reported 10 cases of SOT-associated mucormycosis from their single institution and reviewed 106 other cases in the English-language literature from 1970 through 2002. In this study, the transplanted organs were the kidney (n = 73), heart (n = 16), lung (n = 4), heart and lung (n = 2), liver (n = 19), and kidney and pancreas (n = 2) [8]. The most frequently isolated genera were Rhizopus species (73%) and Mucor species (13%). In 40% of the reported patients, augmented immunosuppression to prevent rejection had been used within 1 month before the onset of mucormycosis [8].
Figure 1.
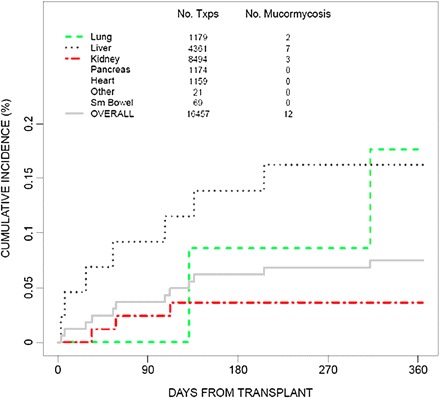
Twelve-month cumulative incidence of mucormycosis among solid-organ transplant (SOT) recipients in the TRANSNET study (adapted from Park et al, Emerg Infect Dis 2011 [18], with permission from B. Park).
Mucormycosis places a particularly high burden on SOT recipients residing in certain countries, as emphasized by a study from Iran that showed that mucormycosis was the most frequent invasive fungal infection in patients receiving SOT in that country [19]. As certain countries have probably higher rates of mucormycosis, the proportion of SOT patients within recent cohorts of patients with mucormycosis varied from 3% to 10% [2, 4, 20]. Of note, in a recent review, up to 24% of 169 patients with healthcare–associated mucormycosis were SOT recipients [21]. In this population, its potential nosocomial acquisition with the allograft as the source of early infection [21], especially in the Middle East and Asia [22], has been documented. In contrast, non–graft-transmitted mucormycosis developed a median of 5 months (range, 1.5–12.0 months) after SOT, with a significantly earlier occurrence in liver transplant recipients [10].
Characteristics of HSCT
Current epidemiological data on mucormycosis in HSCT come from 10 series that each collected at least 10 patients over the past 10 years, including 5 prospective studies (2 from Europe and 3 from the United States) (Table 1). The incidence of mucormycosis in these studies ranged from 0.1% to 2.0%, with the highest rates observed among patients with graft-versus-host disease (GVHD) [23, 26–30]. Uckay et al reported 2 cases from their own center and reviewed 44 previous cases published through 2006 [31]. The 12-month cumulative incidence of mucormycosis from 2001 through 2006 was 0.29% among HSCT recipients in the United States and was higher in cases of allogeneic transplants from HLA-matched unrelated donors [18]. (Figure 2).
Table 1.
Studies Reporting >10 Cases of Allogeneic Hematopoietic Stem Cell Transplant (HSCT) Recipients Who Received a Diagnosis of Mucormycosis
| Study | Type of Study | No. of Patients/Study Period | Outcome |
|---|---|---|---|
| Marr, single institution 2002 [23] | Retrospective | 29/1985–1999 | Median survival: 66 day 1-year survival rate: 20% |
| Roden, literature review 2005 [4] | Retrospective | 44/1940–2003 | Mortality rate: 91% |
| Kontoyiannis, single institution 2005 [24] | Prospective | 13/2002–2004 | Unreported for specific subpopulations |
| Trifilio, multicentric case series 2007 [25] | Retrospective | 34/2002–2005 | Mortality rate: 64% |
| Chamilos, single institution 2008 [5] | Retrospective | 32/1989–2006 | Mortality rate: 56% |
| Bitar, national hospital discharge codes 2009 [7] | Retrospective | 33a/1997–2006 | Mortality rate: 36.4% |
| Neofytos, active surveillance 2009 [26] | Prospective | 12/2004–2007 | 12-week mortality rate: 64.3%b |
| Kontoyiannis, active surveillance 2010 [6] | Prospective | 77a/2001–2006 | 1-year overall survival rate: 28% |
| Rüping, passive surveillance 2010 [20] | Prospective | 12/2006–2009 | Mortality rate: 75% |
| Skiada, passive surveillance 2011 [1] | Prospective | 21/2005–2007 | Mortality rate: 76% |
Including patients with autologous transplantation.
For 12 allogeneic transplant recipients and 6 autologous transplant recipients.
Figure 2.
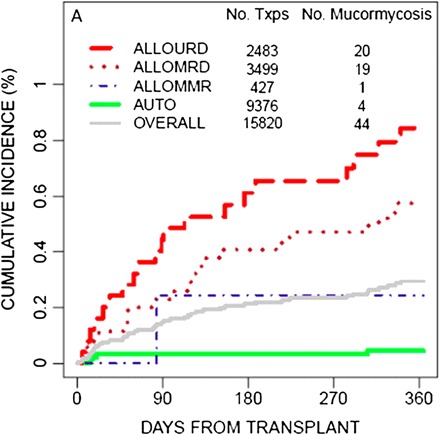
Twelve-month cumulative incidence of mucormycosis for hematopoietic stem-cell transplant (HSCT) recipients, by donor type, in the TRANSNET study. ALLOMMR, allogeneic mismatched related donor; ALLOMRD, allogeneic matched related donor; ALLOURD, allogeneic unrelated donor (adapted from Park et al, Emerg Infect Dis 2011 [18], with permission from B. Park).
In a recent literature review, 5% of patients with mucormycosis had undergone HSCT [4], and among the hematology patients with mucormycosis, the proportion who had undergone allogeneic stem cell transplantation varied from 9% in Italy [27] to 18% in France [2]. Of note, the annual incidence rate for mucormycosis among HSCT recipients increased in France by 15% [7], and a similar trend was seen in a large university hospital in Belgium [32]. More recently, in a French survey, the incidence of mucormycosis was estimated to be 0.4% among 6810 HSCT recipients [33]. Of note, all cases were collected in 11 of 27 participating centers, which is reminiscent of recent TRANSNET data [18] and again strongly suggests that local factors (environmental factors or those related to the transplant procedure, such as the prophylaxis or conditioning regimen used) influence the occurrence of mucormycosis.
Mucormycosis is often a late event after HSCT, with median reported times to occurrence of 135–225 days, which is later than for aspergillosis [6, 28]. Of note, in a recent series, 35% of patients had experienced another invasive fungal infection prior to mucormycosis [33].
RISK FACTORS
Characteristics of SOT
A prospective, matched case-control study in SOT recipients showed that renal failure, diabetes mellitus, and prior voriconazole and/or caspofungin use were associated with a higher risk of mucormycosis [10]. Diabetes likely affects mucormycosis through its impairment of neutrophils and macrophages, which are integral to the innate defense against mucormycosis [34]. Of interest, the case-control study also found that the use of the calcineurin-inhibitor tacrolimus was associated with a 4-fold reduction in the risk of mucormycosis among SOT recipients, even though calcineurin inhibitors are potent immunosuppressive agents [10]. Although calcineurin plays a major role in the pathogenicity of Candida, Cryptococcus, and Aspergillus species, its precise role in the pathogenesis of mucormycosis is not yet fully elucidated. Of note, both calcineurin and mTOR inhibitors have been shown to be active against Mucorales [10, 35]. Calcineurin inhibitors and antifungals were found to have synergistic or additive effects against Mucorales [35], in addition to an increased inhibition of spore germination in vitro, compared with amphotericin B alone (B. Rammaert, unpublished data). Finally, in the case-control study, corticosteroid use alone was not specifically identified as a risk factor for mucormycosis [4, 10].
Iron is a pivotal growth factor for Mucorales, and it also compromises critical host defenses against Mucorales [36]. Thus, liver transplant recipients with iron overload are predisposed to early onset opportunistic infections, including mucormycosis [37].
Characteristics of HSCT
Risk factors for mucormycosis in HSCT recipients have been shown to include severe GVHD, a high steroid dose, previous cytomegalovirus or respiratory viral disease, and increasing age [23, 28]. In one study, 87.5% of mucormycosis cases occurred during chronic extensive GVHD and 48.0% occurred in patients with complicated diabetes [33]. Whether the cytomegalovirus prophylaxis used in HSCT recipients influences the occurrence of mucormycosis remains to be documented, as does the role of iron overload, which remains controversial in these patients [28, 30]. In one study, underlying myelodysplastic syndrome was also emphasized [23]. In addition, female sex has been suggested to be protective against mucormycosis [28], in accordance with the male predominance found recently [33]. Finally, a case-control study found that diabetes mellitus, malnutrition, and voriconazole prophylaxis were risk factors for mucormycosis in hematology patients, including HSCT recipients [24].
However, with the exception of previous voriconazole receipt, the risk factors for mucormycosis and other mold infections in HSCT recipients are consistent in various studies. The precise role of voriconazole in the occurrence of mucormycosis in HSCT patients remains controversial, although several cases have been reported in these patients treated with the drug [18]. In one study, 11 of 29 HSCT recipients with mucormycosis received voriconazole prior to or at the time of mucormycosis diagnosis [33]. However, in a randomized trial, HSCT recipients treated with voriconazole as prophylaxis did not show a higher incidence of mucormycosis than patients who received fluconazole [38]. Nevertheless, there are in vitro and in vivo data indicating that preexposure to voriconazole increases Rhizopus oryzae virulence [39, 40]. Of interest, a few recent mucormycosis cases have also developed as breakthrough infections in HSCT recipients receiving antifungals other than voriconazole, including posaconazole, which is active against several Mucorales species [1, 41].
CLINICAL PRESENTATION
In a recent study, the median time from first symptoms to mucormycosis diagnosis was 6 weeks for SOT patients and 1 week for HSCT recipients [2].
Mucormycosis is localized in 87.1% of SOT recipients, with rhino-sino-orbital localization noticed in 31% of these cases [8]. Among SOT recipients in the TRANSNET study, mucormycosis involved the lungs in 56%, involved the sinuses or skin in 13%, and was disseminated in 9% [42]. In another study involving SOT recipients, pulmonary involvement was found in 53% of patients and consisted of consolidation/mass lesions, nodules, and cavities; lesions were limited to the lungs in nearly 75% of patients with pulmonary involvement, and 41.9%, 29%, and 12.9% were kidney, lung, or heart transplant recipients, respectively [43]. In a recent cohort of 90 SOT recipients with rhino-orbital-cerebral mucormycosis, the maxillary cavities and brain were involved in 80% and 57%, respectively, whereas Rhizopus species were found in 66%. Seventy percent and 12.2% of the SOT patients had received a kidney or liver transplant, respectively [44]. Of note, liver transplantation has been associated with a 5-fold higher risk of disseminated mucormycosis [10].
In patients with graft-transmitted mucormycosis, the clinical features did not differ from those observed in other instances of healthcare–associated mucormycosis [21]. However, graft-transmitted mucormycosis can have a subtle presentation. For example, decreased urinary output and progressive leukocytosis 5–9 days after transplantation were the initial presentation of mucormycosis in 2 recipients of transplanted kidneys from the same donor [45].
Among HSCT recipients with mucormycosis, more than half have pulmonary involvement, 20%–40% have sinus involvement [1, 4, 20, 23, 33], and 10%–15% have involvement of the central nervous system, most frequently in the context of dissemination. Overall, at least 10% of HSCT recipients with mucormycosis have disseminated disease. The main differences in clinical presentation between mucormycosis and invasive aspergillosis in HSCT recipients are the higher frequency of community-acquired sinusitis, the presence of multiple nodules (Figure 3) and pleural effusion, and reverse halo sign seen on computed tomography (CT) scans in mucormycosis [46].
Figure 3.
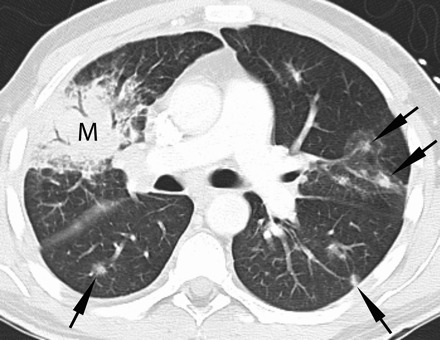
Thirty-eight-year-old man with acute myeloid leukemia, cord blood transplanted with refractory neutropenic fever. Contrast-enhanced chest computed tomography (lung windows) at the level of the main pulmonary artery demonstrates multiple pulmonary nodules (arrows), some of which have a ground glass halo surrounding them and a right upper lobe consolidative mass (M). Biopsy demonstrated fungal hyphae consistent with mucormycosis. Numerous additional nodules were seen throughout the remainder of the lungs (not shown).
DIAGNOSTIC STRATEGY
As soon as a clinical scenario is consistent with mucormycosis, CT of the brain, sinuses, and chest—with or without bronchoscopy when scans of the chest show abnormalities—should be performed [46].
As in other contexts, mucormycosis is diagnosed by direct examination of the involved fluid or tissue and by culture from a sterile site. It should however be noted that direct observation of invasive fungi in a tissue specimen can be suggestive of mucormycosis on the basis of morphology, but this is not definitive criterion because the morphology of fungi in tissue can be difficult for even experienced pathologists. Among 29 recent proven or probable mucormycosis cases in HSCT recipients, direct examination results were positive in 79% and culture results were positive in 86% [33]. Of the 116 SOT recipients with mucormycosis reported in a literature analysis, 61 (52.6%) received a diagnosis on the basis of both biopsy and culture, 45 (38.8%) on the basis of biopsy only, 7 (6%) on the basis of a positive culture result with a compatible clinical presentation, and 3 (2.6%) on the basis of either culture or biopsy (further information was not provided). Although contamination of clinical specimens by Mucorales is possible, positive culture results, especially when repetitive and associated with positive smear results, strongly suggest mucormycosis after transplantation. In a significant number of cases, the culture result is negative or culture is not performed and the only available samples are formalin-fixed paraffin-embedded tissue specimens. In these cases, molecular tools could be used, ideally, to confirm the histopathological diagnosis and/or to identify the fungus to the genus or species level. There is currently no consensus on the best technical parameters to use, and there are no commercially available techniques. Different molecular approaches have been tried. Both formalin-fixed paraffin-embedded and fresh or frozen tissue samples can be used, with a lower yield using the former [47]. The current distribution of Mucorales species in SOT and HSCT recipients in the United States is presented in Figure 4. Finally, negativity for galactomannan and β-glucan reinforce the diagnosis of mucormycosis in the clinical context of invasive fungal infection in transplant recipients [46], although no published data support this statement. Conversely, a positive galactomannan or β-d-glucan result does not exclude the diagnosis of mucormycosis, because of the co-occurrence of fungal infections (up to 45% in a single-center study) [32].
Figure 4.
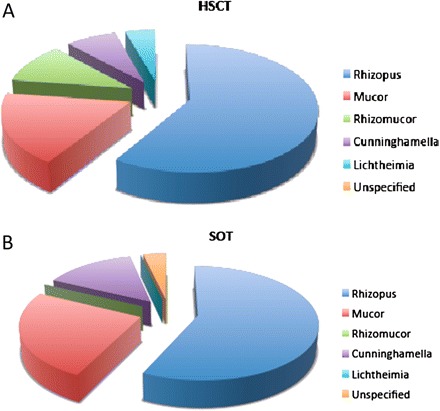
Distribution of Mucorales species among hematopoietic stem-cell transplant (HSCT; A) and solid-organ transplant (SOT; B) recipients in the TRANSNET study (adapted from Park et al, Emerg Infect Dis 2011 [18], with permission from B. Park).
TREATMENT
In the treatment of mucormycosis in SOT and HSCT recipients, improvement of immune and metabolic factors should be routinely considered, by administering tapering doses of steroids and immunosuppressive agents, when feasible, and by controlling hyperglycemia [46].
Because of the rapid growth rate of Mucorales and the knowledge that 84% of patients with pulmonary mucormycosis receive ineffective therapy at the time of diagnosis [48], a rational assumption is that the window of opportunity for effective treatment is even shorter than that for aspergillosis. Indeed, delay in the administration of amphotericin B–based regimens by >5 days is associated with a 2-fold increase in mortality in the hematology context [5].
Polyenes are the preferred therapeutic agents for mucormycosis. Some experimental results suggest that, beyond their improved safety profile, lipid formulations of amphotericin B have better performance than amphotericin B deoxycholate, particularly liposomal amphotericin B [49, 50]. A retrospective study of mucormycosis in 59 patients with hematological disease showed that lipid formulations of amphotericin B resulted in higher recovery rates than did amphotericin B deoxycholate [51]. In addition, the use of lipid formulations of amphotericin B has been associated with better outcomes for SOT recipients with mucormycosis [10]. Therefore, the 2009 recommendations from the European Conference on Infections in Leukemia advocate the use of a lipid formulation of amphotericin B as first-line therapy for mucormycosis [52]. Most experts use liposomal amphotericin B with a usual starting dose of 5 mg/kg/day; this is sometimes escalated to 10 mg/kg/day, if needed, to control the infection.
Animal models have suggested that higher amphotericin B tissue concentrations are needed early in infections for effective treatment of mucormycosis, compared with aspergillosis [53]. This would be consistent with the relative tolerance in vitro of R. oryzae and Cunninghamella species to the fungicidal effects of amphotericin B [54]. A recent study of 28 patients, including 54% with immunosuppressive hematological disorders treated with first-line liposomal amphotericin B (3–14 mg/kg) found a 61% mortality rate [55]. In this context, we have just completed a pilot clinical study using a starting dose of 10 mg/kg/day liposomal amphotericin B for the management of mucormycosis in 40 children and adults, including transplant recipients (AMBIZYGO; available at: http://clinicaltrials.gov/ct2/show/NCT00467883). Hopefully, results of this study should help to delineate the benefits of initial high-dose liposomal amphotericin B in transplant recipients, which currently remains a matter of debate.
After the infection has stabilized, the standard treatment recommendation is oral posaconazole (800 mg/day), for which pharmacokinetic variability can be optimized through careful clinical assessment of gastrointestinal disturbances, improvement in diet, and monitoring of drug levels [46, 52]. To date, posaconazole has largely been used as salvage therapy or in cases of intolerance to amphotericin B, with a success rate of 60% [56]. In SOT recipients, posaconazole as salvage therapy was associated with a successful response in 13 (57%) of 27 patients with invasive fungal infection, including 1 of 2 patients with mucormycosis [45]. It should however be noted that, in vitro, posaconazole is less often active against some common Mucorales species than amphotericin and that posaconazole has important drug-drug interaction issues with immunosuppressants commonly used for SOT and HSCT (including interactions with tacrolimus, cyclosporine, and sirolimus) that can complicate its use.
Of major importance, extensive early surgical debridement is generally recommended in combination with antifungals [52] and was reported to be an independent predictor of successful therapy in SOT recipients with pulmonary or rhino-orbital-cerebral mucormycosis [43, 44]. Anecdotally, in 2 patients with renal mucormycosis after transplantation of kidneys from the same donor, transplant nephrectomy and multiple debridements to remove dead tissue were required to eradicate the infection [45]. Surgery, including pulmonary resection, should also be performed when feasible for HSCT recipients with mucormycosis, but less has been published on this subject.
In cases of treatment failure, combinations of a lipid formulation of amphotericin B at standard doses and an echinocandin [57] might be considered. The role of newer generation iron chelation agents (eg, deferasirox) is controversial. Indeed, the iron chelator deferasirox protects mice from mucormycosis [58]. In contrast, a recent double-blinded, randomized, placebo-controlled, phase 2 safety/exploratory efficacy study of adjunctive deferasirox therapy (20 mg/kg/d for 14 days) for mucormycosis (the Deferasirox-AmBisome Therapy for Mucormycosis study; NCT00419770) has failed to demonstrate a benefit of combination therapy. Instead, there was excess mortality among patients treated with adjunctive deferasirox [59]. Likewise, preclinical data indicate that the combination of a lipid formulation of amphotericin B and posaconazole is unlikely to be effective [60]. Other therapeutic approaches, such as hyperbaric oxygen therapy or the use of new antifungal agents active against Mucorales, such as isavuconazole, have not been clinically validated [52].
The duration of acute phase treatment is not defined. It is at least 6–8 weeks or until resolution of all associated symptoms and findings. Maintenance therapy has to be considered in persistently immunocompromised transplant recipients.
OUTCOMES
Characteristics of SOT
The overall mortality rate among SOT recipients with mucormycosis is generally 38%–48% [4, 8, 61], although some studies have found higher rates. In one study, the mortality rate associated with mucormycosis in SOT recipients exceeded that associated with all other invasive mold infections [9], and a more recent study found that renal failure and disseminated disease independently predicted poor outcomes [10].
Characteristics of HSCT
The median survival of HSCT recipients with mucormycosis has been shown to be <2 months [23, 33], and the disease-related mortality rate has been shown to be at least 75% [1, 4, 6, 20, 23, 24, 26, 33]. One recent study performed in Boston showed that the disease-related mortality rate decreased in recent years among hematology patients and HSCT recipients [62]. Another recent study indicated that young age increased the risk of mucormycosis-attributable mortality among allogeneic HSCT recipients and that male sex and surgery decreased the risk [33]. Finally, another factor associated with death in HSCT recipients with mucormycosis was prior use of voriconazole [18, 33].
CONCLUSIONS
With the knowledge that mucormycosis now represents 2% and 8% of invasive fungal infections in SOT and HSCT recipients, respectively, current risk factors and diagnostic strategies for mucormycosis should be known by transplantation and infectious diseases physicians. Early administration of a lipid formulation of amphotericin B and, when feasible, surgery will optimize the outcome for patients with mucormycosis in the transplant context.
Notes
Acknowledgments.
We thank Professor Edith Marom for providing Figure 3.
Potential conflicts of interest.
All authors: No reported conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Skiada A, Pagano L, Groll A, et al. Zygomycosis in Europe: analysis of 230 cases accrued by the registry of the European Confederation of Medical Mycology (ECMM) Working Group on Zygomycosis between 2005 and 2007. Clin Microbiol Infect. 2011;17:1859–69. doi: 10.1111/j.1469-0691.2010.03456.x. Epub. [DOI] [PubMed] [Google Scholar]
- 2.Lanternier F DE, Morizot G, Elie C, et al. Lortholary O and the French Mycosis study group. A global analysis of mucormycosis in France: the “RetroZygo study” (2005–2007) Clin Infect Dis. 2012;S54:35–43. doi: 10.1093/cid/cir880. [DOI] [PubMed] [Google Scholar]
- 3.Petrikkos G, Skiada A, Lortholary O, Roilides E, Walsh TJ, Kontoyiannis DP. Epidemiology and clinical manifestations of mucormycosis. Clin Infect Dis. 2012;S54:23–34. doi: 10.1093/cid/cir866. [DOI] [PubMed] [Google Scholar]
- 4.Roden MM, Zaoutis TE, Buchanan WL, et al. Epidemiology and outcome of zygomycosis: a review of 929 reported cases. Clin Infect Dis. 2005;41:634–53. doi: 10.1086/432579. [DOI] [PubMed] [Google Scholar]
- 5.Chamilos G, Lewis RE, Kontoyiannis DP. Delaying amphotericin B-based frontline therapy significantly increases mortality among patients with hematologic malignancy who have zygomycosis. Clin Infect Dis. 2008;47:503–9. doi: 10.1086/590004. [DOI] [PubMed] [Google Scholar]
- 6.Kontoyiannis DP, Marr KA, Park BJ, et al. Prospective surveillance for invasive fungal infections in hematopoietic stem cell transplant recipients, 2001–2006: overview of the Transplant-Associated Infection Surveillance Network (TRANSNET) database. Clin Infect Dis. 2010;50:1091–100. doi: 10.1086/651263. [DOI] [PubMed] [Google Scholar]
- 7.Bitar D, Van Cauteren D, Lanternier F, et al. Increasing incidence of zygomycosis (mucormycosis), France, 1997–2006. Emerg Infect Dis. 2009;15:1395–401. doi: 10.3201/eid1509.090334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Almyroudis NG, Sutton DA, Linden P, Rinaldi MG, Fung J, Kusne S. Zygomycosis in solid organ transplant recipients in a tertiary transplant center and review of the literature. Am J Transplant. 2006;6:2365–74. doi: 10.1111/j.1600-6143.2006.01496.x. [DOI] [PubMed] [Google Scholar]
- 9.Husain S, Alexander BD, Munoz P, et al. Opportunistic mycelial fungal infections in organ transplant recipients: emerging importance of non-Aspergillus mycelial fungi. Clin Infect Dis. 2003;37:221–9. doi: 10.1086/375822. [DOI] [PubMed] [Google Scholar]
- 10.Singh N, Aguado JM, Bonatti H, et al. Zygomycosis in solid organ transplant recipients: a prospective, matched case-control study to assess risks for disease and outcome. J Infect Dis. 2009;200:1002–11. doi: 10.1086/605445. [DOI] [PubMed] [Google Scholar]
- 11.Jimenez C, Lumbreras C, Aguado JM, et al. Successful treatment of mucor infection after liver or pancreas-kidney transplantation. Transplantation. 2002;73:476–80. doi: 10.1097/00007890-200202150-00026. [DOI] [PubMed] [Google Scholar]
- 12.Grossi P, Farina C, Fiocchi R, Dalla Gasperina D. Prevalence and outcome of invasive fungal infections in 1963 thoracic organ transplant recipients: a multicenter retrospective study. Italian Study Group of Fungal Infections in Thoracic Organ Transplant Recipients. Transplantation. 2000;70:112–6. [PubMed] [Google Scholar]
- 13.Ko WJ, Chien NC, Chou NK, Wang SS, Chu SH, Chang SC. Infection in heart transplant recipients: seven years' experience at the National Taiwan University Hospital. Transplant Proc. 2000;32:2392–5. doi: 10.1016/s0041-1345(00)01712-7. [DOI] [PubMed] [Google Scholar]
- 14.Bertocchi M, Thevenet F, Bastien O, et al. Fungal infections in lung transplant recipients. Transplant Proc. 1995;27:1695. [PubMed] [Google Scholar]
- 15.Schowengerdt KO, Naftel DC, Seib PM, et al. Infection after pediatric heart transplantation: results of a multiinstitutional study. The Pediatric Heart Transplant Study Group. J Heart Lung Transplant. 1997;16:1207–16. [PubMed] [Google Scholar]
- 16.Ruffini E, Baldi S, Rapellino M, et al. Fungal infections in lung transplantation. Incidence, risk factors and prognostic significance. Sarcoidosis Vasc Diffuse Lung Dis. 2001;18:181–90. [PubMed] [Google Scholar]
- 17.Nampoory MR, Khan ZU, Johny KV, et al. Invasive fungal infections in renal transplant recipients. J Infect. 1996;33:95–101. doi: 10.1016/s0163-4453(96)92986-2. [DOI] [PubMed] [Google Scholar]
- 18.Park BJ PP, Wannemuehler KA, Alexander BP, et al. Invasive non-Aspergillus mold infections in transplant recipients, United States, 2001–2006. Emerg Infect Dis. 2011;17:1855–64. doi: 10.3201/eid1710.110087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Einollahi B, Lessan-Pezeshki M, Pourfarziani V, et al. Invasive fungal infections following renal transplantation: a review of 2410 recipients. Ann Transplant. 2008;13:55–8. [PubMed] [Google Scholar]
- 20.Ruping MJ, Heinz WJ, Kindo AJ, et al. Forty-one recent cases of invasive zygomycosis from a global clinical registry. J Antimicrob Chemother. 2010;65:296–302. doi: 10.1093/jac/dkp430. [DOI] [PubMed] [Google Scholar]
- 21.Rammaert B, Lanternier F, Zahar JR, Dannaoui E, Bougnoux ME, Lecuit M, Lortholary O. Healthcare-associated zygomycosis. Clin Infect Dis. 2012;S54:44–54. doi: 10.1093/cid/cir867. [DOI] [PubMed] [Google Scholar]
- 22.Shoham S, Hinestrosa F, Moore J, Jr, O'Donnell S, Ruiz M, Light J. Invasive filamentous fungal infections associated with renal transplant tourism. Transpl Infect Dis. 2010;12:371–4. doi: 10.1111/j.1399-3062.2010.00498.x. [DOI] [PubMed] [Google Scholar]
- 23.Marr KA, Carter RA, Crippa F, Wald A, Corey L. Epidemiology and outcome of mould infections in hematopoietic stem cell transplant recipients. Clin Infect Dis. 2002;34:909–17. doi: 10.1086/339202. [DOI] [PubMed] [Google Scholar]
- 24.Kontoyiannis DP, Lionakis MS, Lewis RE, et al. Zygomycosis in a tertiary-care cancer center in the era of Aspergillus-active antifungal therapy: a case-control observational study of 27 recent cases. J Infect Dis. 2005;191:1350–60. doi: 10.1086/428780. [DOI] [PubMed] [Google Scholar]
- 25.Trifilio SM, Bennett CL, Yarnold PR, et al. Breakthrough zygomycosis after voriconazole administration among patients with hematologic malignancies who receive hematopoietic stem-cell transplants or intensive chemotherapy. Bone Marrow Transplant. 2007;39:425–9. doi: 10.1038/sj.bmt.1705614. [DOI] [PubMed] [Google Scholar]
- 26.Neofytos D, Horn D, Anaissie E, et al. Epidemiology and outcome of invasive fungal infection in adult hematopoietic stem cell transplant recipients: analysis of Multicenter Prospective Antifungal Therapy (PATH) Alliance registry. Clin Infect Dis. 2009;48:265–73. doi: 10.1086/595846. [DOI] [PubMed] [Google Scholar]
- 27.Pagano L, Valentini CG, Posteraro B, et al. Zygomycosis in Italy: a survey of FIMUA-ECMM (Federazione Italiana di Micopatologia Umana ed Animale and European Confederation of Medical Mycology) J Chemother. 2009;21:322–9. doi: 10.1179/joc.2009.21.3.322. [DOI] [PubMed] [Google Scholar]
- 28.Garcia-Vidal C, Upton A, Kirby KA, Marr KA. Epidemiology of invasive mold infections in allogeneic stem cell transplant recipients: biological risk factors for infection according to time after transplantation. Clin Infect Dis. 2008;47:1041–50. doi: 10.1086/591969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pagano L, Caira M, Nosari A, et al. Fungal infections in recipients of hematopoietic stem cell transplants: results of the SEIFEM B-2004 study–Sorveglianza Epidemiologica Infezioni Fungine Nelle Emopatie Maligne. Clin Infect Dis. 2007;45:1161–70. doi: 10.1086/522189. [DOI] [PubMed] [Google Scholar]
- 30.Maertens J, Demuynck H, Verbeken EK, et al. Mucormycosis in allogeneic bone marrow transplant recipients: report of five cases and review of the role of iron overload in the pathogenesis. Bone Marrow Transplant. 1999;24:307–12. doi: 10.1038/sj.bmt.1701885. [DOI] [PubMed] [Google Scholar]
- 31.Uckay I, Chalandon Y, Sartoretti P, et al. Invasive zygomycosis in transplant recipients. Clin Transplant. 2007;21:577–82. doi: 10.1111/j.1399-0012.2007.00684.x. [DOI] [PubMed] [Google Scholar]
- 32.Saegeman V, Maertens J, Meersseman W, Spriet I, Verbeken E, Lagrou K. Increasing incidence of mucormycosis in University Hospital, Belgium. Emerg Infect Dis. 2010;16:1456–8. doi: 10.3201/eid1609.100276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Xhaard AL, Porcher R, Dannaoui E, et al. Mucormycosis after allogeneic hematopoietic stem cell transplantation: a French Multicenter Cohort Study (2003–2008) Clin Microbiol Infect; revised; [DOI] [PubMed] [Google Scholar]
- 34.Lanternier F, Lortholary O. Zygomycosis and diabetes mellitus. Clin Microbiol Infect. 2009;15(Suppl 5):21–5. doi: 10.1111/j.1469-0691.2009.02975.x. [DOI] [PubMed] [Google Scholar]
- 35.Dannaoui E, Schwarz P, Lortholary O. In vitro interactions between antifungals and immunosuppressive drugs against zygomycetes. Antimicrob Agents Chemother. 2009;53:3549–51. doi: 10.1128/AAC.00184-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Singh N, Sun HY. Iron overload and unique susceptibility of liver transplant recipients to disseminated disease due to opportunistic pathogens. Liver Transpl. 2008;14:1249–55. doi: 10.1002/lt.21587. [DOI] [PubMed] [Google Scholar]
- 37.Singh N, Wannstedt C, Keyes L, et al. Hepatic iron content and the risk of Staphylococcus aureus bacteremia in liver transplant recipients. Prog Transplant. 2007;17:332–6. doi: 10.1177/152692480701700412. [DOI] [PubMed] [Google Scholar]
- 38.Wingard JR, Carter SL, Walsh TJ, et al. Randomized, double-blind trial of fluconazole versus voriconazole for prevention of invasive fungal infection after allogeneic hematopoietic cell transplantation. Blood. 2010;116:5111–8. doi: 10.1182/blood-2010-02-268151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lewis RE, Liao G, Wang W, Prince RA, Kontoyiannis DP. Voriconazole pre-exposure selects for breakthrough mucormycosis in a mixed model of Aspergillus fumigatus-Rhizopus oryzae pulmonary infection. Virulence. 2011;2:348–55. doi: 10.4161/viru.2.4.17074. [DOI] [PubMed] [Google Scholar]
- 40.Lamaris GA, Ben-Ami R, Lewis RE, Chamilos G, Samonis G, Kontoyiannis DP. Increased virulence of Zygomycetes organisms following exposure to voriconazole: a study involving fly and murine models of zygomycosis. J Infect Dis. 2009;199:1399–406. doi: 10.1086/597615. [DOI] [PubMed] [Google Scholar]
- 41.Schlemmer F, Lagrange-Xelot M, Lacroix C, de La Tour R, Socie G, Molina JM. Breakthrough Rhizopus infection on posaconazole prophylaxis following allogeneic stem cell transplantation. Bone Marrow Transplant. 2008;42:551–2. doi: 10.1038/bmt.2008.199. [DOI] [PubMed] [Google Scholar]
- 42.Pappas PG, Alexander BD, Andes DR, et al. Invasive fungal infections among organ transplant recipients: results of the Transplant-Associated Infection Surveillance Network (TRANSNET) Clin Infect Dis. 2010;50:1101–11. doi: 10.1086/651262. [DOI] [PubMed] [Google Scholar]
- 43.Sun HY, Aguado JM, Bonatti H, et al. Pulmonary zygomycosis in solid organ transplant recipients in the current era. Am J Transplant. 2009;9:2166–71. doi: 10.1111/j.1600-6143.2009.02754.x. [DOI] [PubMed] [Google Scholar]
- 44.Sun HY, Forrest G, Gupta KL, et al. Rhino-orbital-cerebral zygomycosis in solid organ transplant recipients. Transplantation. 2010;90:85–92. doi: 10.1097/tp.0b013e3181dde8fc. [DOI] [PubMed] [Google Scholar]
- 45.Alexander BD, Schell WA, Siston AM, et al. Fatal Apophysomyces elegans infection transmitted by deceased donor renal allografts. Am J Transplant. 2010;10:2161–7. doi: 10.1111/j.1600-6143.2010.03216.x. [DOI] [PubMed] [Google Scholar]
- 46.Kontoyiannis DP, Lewis RE. How I treat mucormycosis. Blood. 2011;118:1216–24. doi: 10.1182/blood-2011-03-316430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Garcia-Hermoso D, Dannaoui E, Lortholary O, Dromer F. Agents of systemic and subcutaneous mucormycosis and entomophthoromycosis. In: American Society for Microbiology, ed. Manual of clinical microbiology, 10th ed. Washington, DC: 2011, chapter 119. [Google Scholar]
- 48.Lass-Florl C, Resch G, Nachbaur D, et al. The value of computed tomography-guided percutaneous lung biopsy for diagnosis of invasive fungal infection in immunocompromised patients. Clin Infect Dis. 2007;45:e101–4. doi: 10.1086/521245. [DOI] [PubMed] [Google Scholar]
- 49.Ibrahim AS, Avanessian V, Spellberg B, Edwards JE., Jr Liposomal amphotericin B, and not amphotericin B deoxycholate, improves survival of diabetic mice infected with Rhizopus oryzae. Antimicrob Agents Chemother. 2003;47:3343–4. doi: 10.1128/AAC.47.10.3343-3344.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ibrahim AS, Gebremariam T, Husseiny MI, et al. Comparison of lipid amphotericin B preparations in treating murine zygomycosis. Antimicrob Agents Chemother. 2008;52:1573–6. doi: 10.1128/AAC.01488-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pagano L, Offidani M, Fianchi L, et al. Mucormycosis in hematologic patients. Haematologica. 2004;89:207–14. [PubMed] [Google Scholar]
- 52.Skiada A, Lanternier F, Groll A, et al. Diagnosis and treatment of zygomycosis: guidelines from the 3rd European Conference on Infections in Leukemia (ECIL 3) Submitted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lewis RE, Liao G, Hou J, Chamilos G, Prince RA, Kontoyiannis DP. Comparative analysis of amphotericin B lipid complex and liposomal amphotericin B kinetics of lung accumulation and fungal clearance in a murine model of acute invasive pulmonary aspergillosis. Antimicrob Agents Chemother. 2007;51:1253–8. doi: 10.1128/AAC.01449-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lass-Florl C, Mayr A, Perkhofer S, et al. Activities of antifungal agents against yeasts and filamentous fungi: assessment according to the methodology of the European Committee on Antimicrobial Susceptibility Testing. Antimicrob Agents Chemother. 2008;52:3637–41. doi: 10.1128/AAC.00662-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Shoham S, Magill SS, Merz WG, et al. Primary treatment of zygomycosis with liposomal amphotericin B: analysis of 28 cases. Med Mycol. 2010;48:511–7. doi: 10.3109/13693780903311944. [DOI] [PubMed] [Google Scholar]
- 56.van Burik JA, Hare RS, Solomon HF, Corrado ML, Kontoyiannis DP. Posaconazole is effective as salvage therapy in zygomycosis: a retrospective summary of 91 cases. Clin Infect Dis. 2006;42:e61–5. doi: 10.1086/500212. [DOI] [PubMed] [Google Scholar]
- 57.Reed C, Bryant R, Ibrahim AS, et al. Combination polyene-caspofungin treatment of rhino-orbital-cerebral mucormycosis. Clin Infect Dis. 2008;47:364–71. doi: 10.1086/589857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ibrahim AS, Gebermariam T, Fu Y, et al. The iron chelator deferasirox protects mice from mucormycosis through iron starvation. J Clin Invest. 2007;117:2649–57. doi: 10.1172/JCI32338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Spellberg B, Ibrahim AS, Chin-Hong PV, et al. The Deferasirox-AmBisome Therapy for Mucormycosis (DEFEAT Mucor) study: a randomized, double-blinded, placebo-controlled trial. J Antimicrob Chemother. 2012;67:715–22. doi: 10.1093/jac/dkr375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ibrahim AS, Gebremariam T, Schwartz JA, Edwards JE, Jr, Spellberg B. Posaconazole mono- or combination therapy for treatment of murine zygomycosis. Antimicrob Agents Chemother. 2009;53:772–5. doi: 10.1128/AAC.01124-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Chakrabarti A, Chatterjee SS, Das A, et al. Invasive zygomycosis in India: experience in a tertiary care hospital. Postgrad Med J. 2009;85:573–81. doi: 10.1136/pgmj.2008.076463. [DOI] [PubMed] [Google Scholar]
- 62.Hammond S BL, Marty F. Mortality in hematologic malignancies and hematopoietic stem cell transplant patients with mucormycosis 2001–2009. Antimicrob Agents Chemother. 2011;55:5018–21. doi: 10.1128/AAC.00536-11. [DOI] [PMC free article] [PubMed] [Google Scholar]


