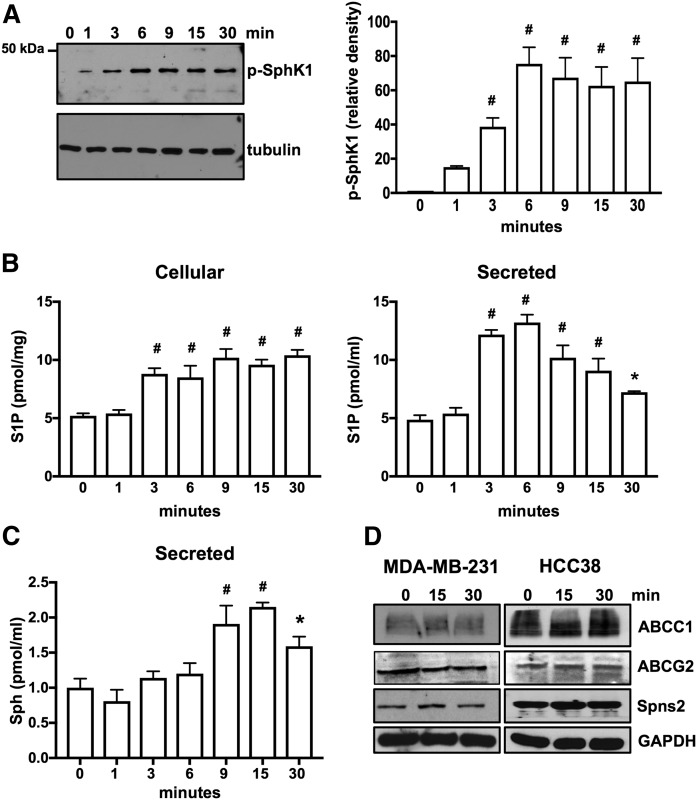
Fig. 2.
E2 induces phosphorylation of SphK1 for production and secretion of S1P in HCC38 breast cancer cells that express only ERα36. A, B: Serum-starved HCC38 cells were treated with E2 (100 nM) for the indicated time. A: Proteins in cell lysates were separated by SDS-PAGE and immunoblotted with anti-phospho-SphK1 Ab. Blots were stripped and reprobed with antitubulin to show equal transfer and loading. Phospho-SphK1 was quantified by densitometry and data expressed as relative density of p-SphK1 normalized to tubulin. B, C: Cellular S1P and S1P released into the medium during 30 min secretion assays as well as sphingosine (Sph) were measured by LC/ESI/MS/MS. Data are mean ± SD. * P ≤ 0.05; # P < 0.001 (compared with 0 time). D: Serum-starved MDA-MB-231 and HCC38 cells were treated with E2 (100 nM) for the indicated time, and cell lysate proteins were immunoblotted with the indicated Abs. Blots were stripped and reprobed with anti-GAPDH to show equal transfer and loading.