Abstract
Glycosyl hydrolases (GHs) are carbohydrate-active enzymes that hydrolyze a specific β-glycosidic bond in glycoconjugate substrates; β-glucosidases degrade glucosylceramide, a ubiquitous glycosphingolipid. GHs are grouped into structurally similar families that themselves can be grouped into clans. GH1, GH5, and GH30 glycosidases belong to clan A hydrolases with a catalytic (β/α)8 TIM barrel domain, whereas GH116 belongs to clan O with a catalytic (α/α)6 domain. In humans, GH abnormalities underlie metabolic diseases. The lysosomal enzyme glucocerebrosidase (family GH30), deficient in Gaucher disease and implicated in Parkinson disease etiology, and the cytosol-facing membrane-bound glucosylceramidase (family GH116) remove the terminal glucose from the ceramide lipid moiety. Here, we compare enzyme differences in fold, action, dynamics, and catalytic domain stabilization by binding site occupancy. We also explore other glycosidases with reported glycosylceramidase activity, including human cytosolic β-glucosidase, intestinal lactase-phlorizin hydrolase, and lysosomal galactosylceramidase. Last, we describe the successful translation of research to practice: recombinant glycosidases and glucosylceramide metabolism modulators are approved drug products (enzyme replacement therapies). Activity-based probes now facilitate the diagnosis of enzyme deficiency and screening for compounds that interact with the catalytic pocket of glycosidases. Future research may deepen the understanding of the functional variety of these enzymes and their therapeutic potential.
Keywords: cerebrosides, cholesterol, glycolipids, Gaucher disease, enzymology, crystal, Parkinson disease
Glycoconjugates play essential roles in diverse biological processes and abnormalities and have been linked to multiple pathologies. The staggering structural diversity of glycoconjugates stems from the almost infinite possible combinations by which multiple monosaccharide building blocks may be linked with each other (1). For instance, 1,056 unique trisaccharides can be formed just from 3 different monosaccharides. Polysaccharides are very stable compounds: their spontaneous hydrolysis takes place at a rate of 10−15 s−1, corresponding to a half-life of 4.7 million years (2). To allow for the efficient metabolism of glycoconjugates, enzymes have evolved as specialized catalysts. In most organisms, an estimated 1% to 3% of the genes encode carbohydrate-active enzymes (3). Among these are glycoside hydrolases (GHs) that can enhance the rate of hydrolysis of specific carbohydrate glycosidic bonds in glycoconjugates more than 1017 times (2). These GH enzymes show marked specificity regarding number, position, and configuration of the hydroxyl groups in their substrate sugar. They are widely applied in biotechnology, for example, in biofuel production, paper pulp bleaching, and the food industry (4, 5). Likewise, specific GH inhibitors are extensively used as agrochemicals and therapeutic agents (6–8). Abnormalities in GHs underlie metabolic disorders in humans, for instance, inherited lysosomal storage disorders and lactose intolerance (9, 10). GH enzymes differ in substrate specificity, mode of enzymatic attack (exo- vs. endoenzymes), and stereochemical mechanism and outcome (retaining vs. inverting enzymes) (11). Over the last 20 years, the Carbohydrate-Active Enzymes database (http://www.cazy.org) has been developed. It distinguishes families of structurally related catalytic enzymes that degrade, modify, or create glycosidic bonds and identifies evolutionarily related families of GHs using the classification introduced by Bernard Henrissat (12, 13). At present, >140 discrete GH families are known (11).
All eukaryote cells contain the glycosphingolipid β-glucosylceramide (GlcCer). In humans, the metabolism of GlcCer implies the removal of the terminal glucose from the ceramide lipid backbone by the lysosomal enzyme glucocerebrosidase (GBA1; family GH30), the cytosol-facing membrane-bound glucosylceramidase (GBA2; family GH116), and cytosolic GBA3 (family GH1) (14). Endoglycosylceramidases (family GH5) from lower organisms cleave the same linkage in complex glycosphingolipids, releasing the oligosaccharide in the process. A relatively common inherited lysosomal storage disorder, Gaucher disease, results from mutations in the GBA gene (15, 16). Most common are mutations that result in impaired folding and lysosomal stability of GBA1 (14, 17). Moreover, deficiency of this enzyme has been implicated in the etiology of Parkinson disease (18, 19). Inherited deficiency of GBA2 results in spastic paraplegia and cerebellar ataxia (20–24).
This review focuses on the retaining β-glucosidases involved in the metabolism of GlcCer and complex glycosphingolipids. Table 1 presents an overview of the discussed β-glycosylceramidases and their GH families. Addressed are their fold topology, dynamics, mode of action, and catalytic domain stabilization by binding site occupancy. The more recent translation of fundamental knowledge on these glycosidases and their selective inhibitors to applications in industry and the clinic are discussed.
TABLE 1.
β-Glycosylceramidases
| Fold | Clan | GH Family | β-Glycosylceramidase Members |
| (β/α)8 | A | GH1 | LPH; GBA3 |
| GH5 | EGCII; EGCI; endogalactosylceramidase EGALC; glucocerebrosidase EGCrP1; steryl-β-glucosidase EGCrP2; steryl-β-glucosidase EGH1 | ||
| GH30 | GBA1 | ||
| GH59 | GALC | ||
| (α/α)6 | O | GH116 | GBA2 |
GLYCOSYLCERAMIDASES: CATALYTIC MECHANISM
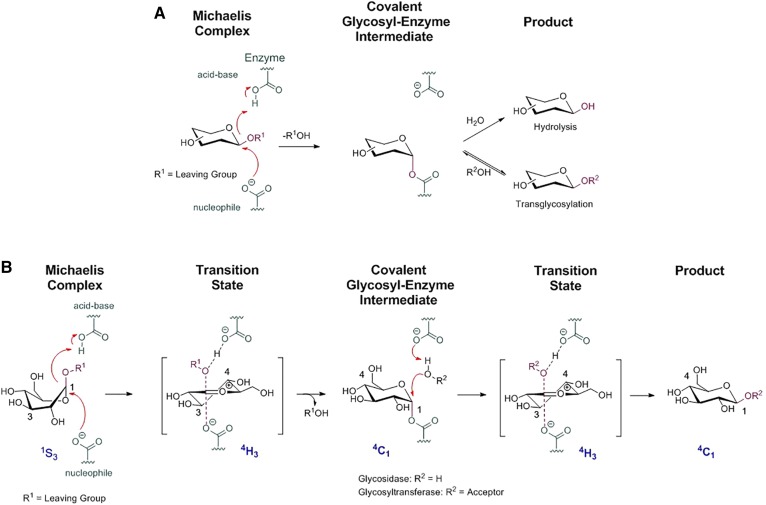
Retaining β-glycosidases generally employ the Koshland double-displacement mechanism with a catalytic nucleophile and acid/base for catalysis (25). The first step is a nucleophilic attack by the catalytic amino acid to the anomeric carbon of the glycosidic substrate. Concomitantly with the nucleophilic attack, a proton transfer from the acid/base residue followed by departure of the leaving group from the aglycon site occurs during the first transition state, from which an enzyme-glycoside covalent intermediate emerges. In the second step, an activated water molecule acts as a nucleophile with the assistance of the general acid/base to deglycosylate the nucleophile residue. The product is released from the enzymatic pocket through a second oxocarbenium ion transition state, and a new catalytic cycle can take place (Fig. 1). Thus, the reaction involves two transient oxocarbenium ion-like states, and the sugar substrate adopts different itineraries depending on its pyranose ring configuration (25). In the case of GH1, GH5, and GH30 enzymes, the substrate itinerary is supposed to follow the 1S3 → 4H3 → 4C1 → 4H3 → 4C1 pathway for the Michaelis complex → transition state → covalent intermediate → transition state → product trajectory (26, 27). Several retaining β-glycosidases are reported to also be able to transglycosylate when provided with a suitable aglycon acceptor (Fig. 1) (28). Transglycosylation capacity has been found to be influenced by temperature, pH, and the presence of organic solvent (29–31). This reaction has been successfully applied to synthesize oligosaccharides and glycoconjugates. The transglycosylation capacity of (mutant) β-glycosidases, in combination with their high regio- and stereospecificity, makes them an attractive instrument for synthesizing complex carbohydrates.
Fig. 1.
A: General hydrolysis and transglycosylation mechanism. B: Koshland double-displacement mechanism and substrate itinerary of retaining β-glucosidases.
GLYCOSYLCERAMIDASES OF FAMILY GH5 AND GH30
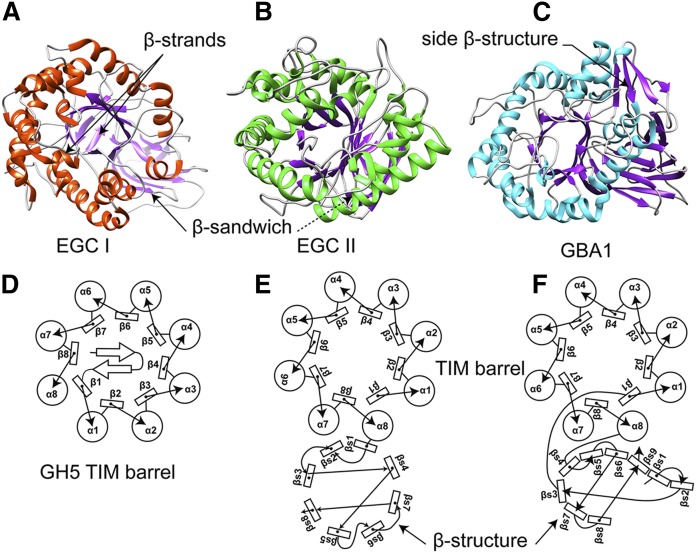
All GH5 and GH30 glycosidases have an (α/β)8 TIM barrel catalytic domain with two conserved carboxylic acid residues between β-strands 4 and 7, serving as the nucleophile and acid/base catalytic dyad. The distance between these two catalytic residues is highly conserved, often between 5 and 5.5 Å between the Oε1 and Oε2 atoms of the nucleophile and acid/base glutamic acid residues, respectively (32). GH5 and GH30 glycosidases comprise fungal, bacterial, and eukaryotic β-1-4 glucanases, β-1-4 mannases, β-1-4 xylanases, cellulases, and glucosylceramidases (33, 34). GH5 glycosidases have been further classified into 53 subfamilies, providing a more accurate prediction of function of yet uncharacterized proteins (34). A new classification approach has led to the transfer of five GH5 protein subgroups to GH30 group 2, including the lysosomal enzyme GBA1 (Fig. 2C). The GH5 and GH30 glycosidases resemble each other regarding protein structure and substrate specificities but show differences in topology (33, 35). For instance, one characteristic of the GH30 enzymes is the fusion of the (α/β)8 barrel catalytic domain with a β-structure consisting of an immunoglobulin-like fold (Fig. 2F). This β-structure, poorly conserved in GH30 glycosidases, is absent in GH5 enzymes (Fig. 2D). Characteristically, the TIM barrel of most GH5 enzymes is sealed with a cap-like structure that does not occur in GH30 enzymes. A special case stands for endoglycoceramidase II (EGCII) from Rhodococcus sp. that removes the entire oligosaccharide from gangliosides such as GM3 and GM1 (Fig. 2B) (36). EGCII has two fold domains, a catalytic TIM barrel adjoined to a β-sandwich domain, as in GH30 members. However, the β-structure domain differs from the ones observed in most GH30 enzymes in that it is composed of only eight β-strands in a barrel geometry (Fig. 2E). The TIM barrel of EGCII is not capped by the small β-strand sheet observed in most other GH5 family members (37). Unlike EGCII, endoglycoceramidase I (EGCI) (another endoglycoceramidase from R. equi with broader substrate specificity than EGCII) displays a typical GH5 TIM barrel fold sealed with two β-strands at the noncatalytic face of the domain. However, in addition, the TIM barrel of this enzyme is also fused to a β-sandwich structure (38) (Fig. 2A). Thus, EGCI is a typical GH5 enzyme, while EGCII shows structural features of members of both GH5 and GH30 families. The evolutionary relationship between these enzymes remains unclear. Of note, the existence and characterization of an animal endoglycoceramidase in the animal kingdom was first described for leeches by Li et al. (39, 40). The same researchers later reported on a similar enzyme in earthworms (41), clams (42), and oysters (43). In humans, no endoglycoceramidase is characterized yet, although an endohydrolysis activity toward gangliosides has been noted for human cancer cells and tissues of other mammals (44, 45).
Fig. 2.
GH5 and GH30 general fold and secondary structure arrangement. A: EGCI 3D structure (PDB ID: 5j7z) (38). B: EGCII 3D structure (PDB ID: 2osx) (37). C: GBA1 3D structure (PDB ID:2v3e) (83). D: Diagram of the GH5 family members’ secondary structure organization (33). E: EGCII secondary structure arrangement (PDB: 2osx) (37). F: GBA1 (PDB: 1ogs) secondary structure element of GH30 members (33). β# refers to TIM barrel domain β-strand number, and βs# refers to a β-strand number of the β-side domain.
In addition to its capacity to hydrolyze, GBA1 may act in vivo as transglucosidase, generating β-glucosylcholesterol (46). The accumulation of cholesterol in lysosomes, as occurs in Niemann-Pick type C or experimentally induced with U18666A, promotes the formation of glucosylated sterols by GBA1 (47) Moreover, GH5 EGCII has been engineered by mutagenesis into a highly efficient transglucosidase applicable for carbohydrate synthesis (48, 49).
Other glycosylceramidases classified in family GH5 are bacterial (Rhodococcus sp.) endogalactosylceramidase, first named EGCIII (50, 51); Cryptococcus neoformans glucocerebrosidase EGCrP1 (52); steryl-β-glucosidase EGCrP2 (53); and Saccharomyces cerevisiae steryl-β-glucosidase EGH1 (54). The endoglycoceramidases and EGPr1 degrade only glycolipids with a ceramide moiety. In contrast, EGH1 and EGCPr2 are reported to hydrolyze both glucosylceramide and steryl-β-glucosides (53). No crystal structure of these enzymes is available yet.
GLYCOSYLCERAMIDASES OF FAMILY GH116
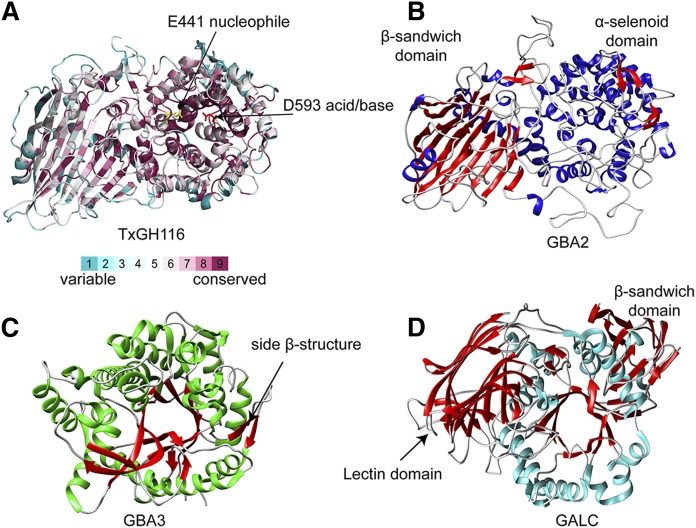
The GH116 family comprises enzymes with diverse specificities and includes the glucosylceramidase GBA2 (55). The partial homology with β-xylosidase/β-glucosidase SSO1353 of Sulfolobus solfataricus assisted the identification of E527 as the nucleophile and D677 as the acid/base in GBA2 (55, 56). At present, no 3D structure of GBA2 is available, but recently such a structure was reported for a GH116 β-glucosidase of Thermoanaerobacterium xylanolyticum (TxGH116), alone and in complex with diverse ligands (57). The TxGH116 structure consists of an N-terminal domain, primarily formed by a two-sheet β-sandwich, and a catalytic C-terminal (α/α)6 solenoid domain (Fig. 3A). The putative catalytic nucleophile is at the end of a long loop between the first and second α-helix of the C-terminal domain, while the putative catalytic acid/base is in a long loop between the fifth and sixth helix of the solenoid, containing the binding site for a structural Ca2+ ion. The N-terminal β-sandwich is tightly associated with the catalytic domain and contributes to the substrate binding cleft and unusual orientation of the acid/base residue. The TxGH116 structures allowed the identification of the glucoside binding- and active-site residues, which are conserved with GBA2. Mutagenic analysis of TxGH116 and structural modeling of GBA2 (Fig. 3B) provided a rationale for pathogenic missense mutations of GBA2 (57). The amino acid sequence of the GH116 fold is highly conserved within the N-terminal β-sandwich and the catalytic C-terminal (α/α)6 solenoid domains (Fig. 3A).
Fig. 3.
GH1, GH59, and GH116 general fold topology. A: G116 TxGH116 structure (PDB ID: 5bvu) (57) with the position of the conserved amino residues of GH116 members depicted on the TxGH116 structure based on sequence alignment using the ConSurf Server. B: GBA2 model based on the TxGH116 structure. C: GH1 glycosylceramidase GBA3 structure (PDB ID: 2e9m) (66). D: GH59 GALC (PDB ID: 4ccc) (73).
Studies on the degradation of GlcCer in β-glucosidase-deficient cells rendered the first indication for the existence of GBA2 (20). The enzyme is synthesized as cytosolic protein and rapidly associates to the cytosolic leaflet of membranes with its catalytic pocket inserted in the lipid layer (20). Its activity is lost upon extraction from membranes with detergents, hampering further characterization of the enzyme. Independently, Yildiz et al. (58) and Boot et al. (59) cloned the gene encoding GBA2 (locus 1p13). GBA2 shows prominent transglucosylase capacity (see section below) and is largely responsible for the (reversible) formation of β-glucosylcholesterol from GlcCer in cells and tissues (47). Given its membrane-embedded pocket, GBA2 seems ideally positioned for the transfer of a glucose between membrane lipids and steroids. Different subcellular localizations of GBA2 have been observed, ranging from endosomes to the Golgi apparatus and ER (60). The physiological function of the highly conserved enzyme is enigmatic. The inhibition of GBA2 in Gaucher disease and Niemann-Pick type C patients treated with N-butyldeoxynojirimycin (NB-DNJ; miglustat) seems not to cause major complications, whereas some individuals with inherited GBA2 deficiency develop spastic paraplegia and cerebellar ataxia (21–24). Mice lacking GBA2 develop normally and show no overt abnormality, except for incidences of male infertility (61). The interaction of GBA2 with actin has been put forward as an explanation for the defective spermatogenesis in GBA2-deficient mice (62). On the other hand, excessive activity of the enzyme has been demonstrated to be toxic in Gaucher disease and other inherited lysosomal glycosphingolipid storage diseases (47, 55).
OTHER PROTEINS OF FAMILY GH1 RESEMBLING GLYCOSYLCERAMIDASES
Based on their primary structure alignment, some proteins of the GH1 family have been considered to be glucosylceramidases. These GH1 proteins contain a β-glucosidase or β-glucosidase-like TIM barrel domain. Among them is lactase phlorizin hydrolase (LPH) (63). LPH is an intestinal 130–160 kDa glycoprotein that is produced as a propeptide with four homologous domains, two of which are catalytically active. One of these domains shows β-glucosidase activity and is active toward phlorizin (plant phloretin-2′-β-d-glucopyranoside) and possibly glucosylceramide; the other domain shows β-galactosidase activity and is active toward lactose (β-galactosyl-d-glucose). Both catalytic domains are assumed to adopt an (α/β)8 TIM barrel fold. Detailed structure information stemming from crystallography is still required to shed light on the interplay between the activities of both catalytic domains. LPH deficiency causes congenital disaccharide intolerance II, a gastrointestinal disorder characterized by severe diarrhea in infants when fed with lactose as abundantly present in milk (64). The GH1 family also includes the enzyme GBA3, a cytosolic β-glucosidase expressed in some cell types. This enzyme has a broad substrate specificity toward aryl glycosides and dietary flavonoids and shows very low activity toward glycosylceramides and glycosylsphingosine in vitro (65). GBA3 contains no glycans, has a molecular weight of 53 kDa, and shows a near-neutral pH optimum. It has also been named Klotho-related protein (59). This name refers to structural similarities to proteins of the Klotho family, none of which have been found to exert significant glycosylceramidase activity. The 3D structure of GBA3 was solved in its free from and in complex with ligands, revealing a typical (α/β)8 TIM barrel domain of the GH1 family in which E167 and E373 of strands 4 and 7 are assigned as the acid/base and nucleophile catalysts, respectively (Fig. 2C). Next to the TIM barrel of GBA3 there are two β-strands that are somewhat reminiscent to the side β-structure in GBA1 (66–68).
GALACTOSYLCERAMIDASE OF FAMILY GH59
Galactocerebrosidase (GALC) is encoded by the GALC gene at locus 14q31 (69). GALC is synthesized as a 669-amino acid polypeptide with a regular N-terminal signal sequence and 6 glycans and transported to lysosomes by mannose-6-phosphate (M6P) receptors (70). The 80–85 kDa precursor GALC is converted in lysosomes to the active form consisting of two subunits of 50–52 kDa and 30 kDa (70). GALC employs the double-displacement mechanism with E258 as the nucleophile and E182 as the general acid/base (71). This enzyme shows optimal activity at pH 4.6 coinciding with the lysosomal milieu. The catalytic activity toward galactosylceramide is improved by interaction with the small accessory proteins saposin A and saposin C, which are generated by proteolytic processing in lysosomes of the precursor prosaposin (72). The 3D structure of GALC has been solved by X-ray crystallography, revealing a catalytic TIM barrel structure (Fig. 3D) (71, 73). The domain architecture of GALC is unique with a previously uncharacterized lectin domain not observed in other hydrolases. This has led to its classification into a specific family, GH59. All three domains of GALC contribute to the substrate binding pocket, and disease-causing mutations are widely distributed throughout the protein (71, 74).
Of note, the seminal work by Suzuki (75) has provided evidence for a key role of elevated galactosylsphingosine in the pathology of Krabbe disease. Galactosylsphingosine (deacylated galactosylceramide) is normally degraded by GALC. In Krabbe patients galactosylsphingosine is excessive, possibly also by active formation from accumulating galactosylceramide through the action of lysosomal acid ceramidase (76, 77). In Gaucher disease and Fabry disease, excessive formation of potential pathogenic glucosylsphingosine and globotriaosylsphingosine respectively occurs (78). This is likely mediated by the metabolism of accumulating intralysosomal glucosylceramide and globotriaosylceramide by acid ceramidase (79).
GLYCOSYLCERAMIDASES: PRESENCE OF N-GLYCANS
Depending on their cellular topology, β-glycosylceramidases may have N-linked glycans. All lysosomal enzymes are equipped with N-glycans that may play a role in folding, intracellular trafficking, and delivery to lysosomes (14). For example, lysosomal GALC has six glycans that acquire M6P moieties in the Golgi apparatus, allowing subsequent M6P receptor-mediated routing of newly synthesized enzymes to lysosomes (80). The reuptake of secreted GALC upon binding to M6P receptors at the cell surface also ensures the delivery of enzymes to lysosomes. This pathway is actually exploited in enzyme replacement therapies for several lysosomal storage disorders (see Therapeutic Enzymes section). The N-glycans of the lysosomal enzyme GBA1 do not acquire M6P (81); newly formed GBA1 is transported to lysosomes by binding to lysosome membrane protein 2 in the ER (82). Of note, not all β-glycosylceramidases have N-linked glycans: for example, human GBA2 and GBA3, being cytosolic proteins, lack these glycans.
GLYCOSYLCERAMIDASES: POCKET ARCHITECTURE
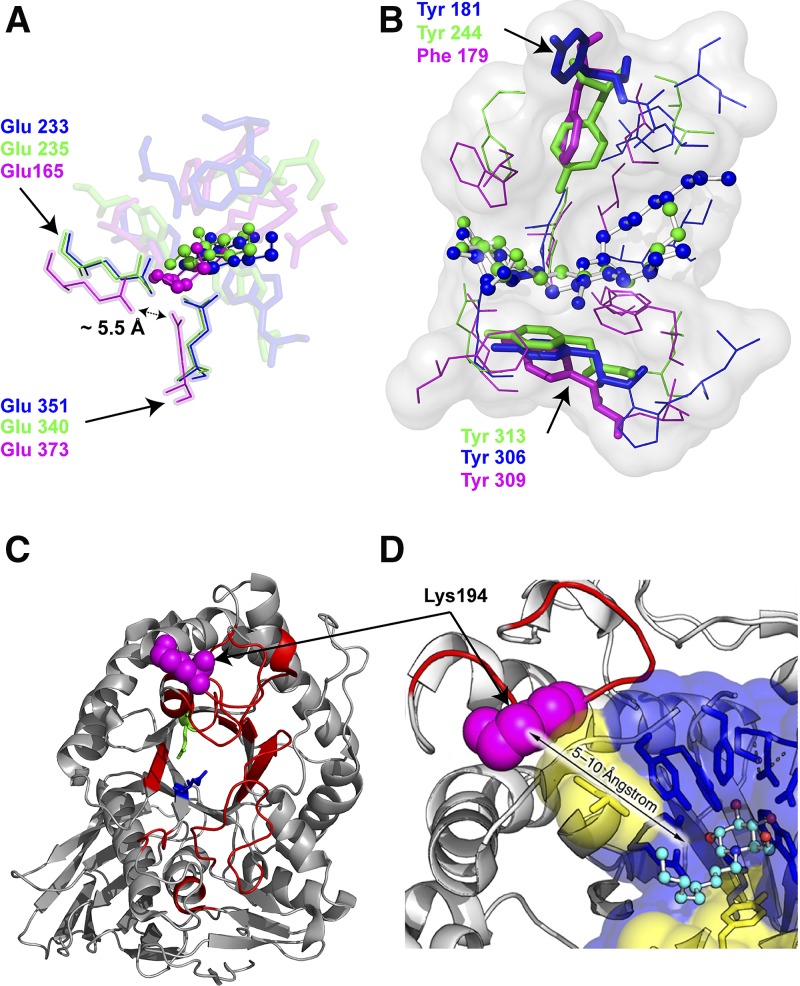
All glycosylceramidases hydrolyze glycosphingolipids despite apparent differences in fold topologies. The enzymes show a glycon binding site where the hydrophilic sugar moiety is accommodated and an aglycon binding site where the hydrophobic ceramide is positioned. The superposition of EGCII (GH5) in complex with GM3 [Protein Data Bank (PDB) ID: 2osx] (37), GBA1 (GH30) in complex with N-nonyldeoxynojirimycin (NN-DNJ) (PDB ID: 2v3e) (83), and GBA3 in complex with galactose and fatty acids (PDB ID: 2e9m) (66) reveals remarkable overlap of nucleophile and acid base catalytic residues in the glycon binding site as well as a comparable location of the glycon and aglycon binding sites (Fig. 4A, B). The substrate sugars have a similar position and orientation in the glycon binding site stabilized by hydrogen bonds between protein side-chain residues and substrate. The wider glycon binding site of EGCII allows its activity toward glycosphingolipids with a large oligosaccharide moiety. The hydrophobic amino acid residues of the aglycon site are not strictly conserved, except for Tyr306 (EGCII), Tyr313 (GBA1), and Tyr309 (GBA3), as well as Tyr181 (EGCII), Tyr244 (GBA1), and Phe179 (GBA3), residing close to the catalytic dyad in a position similar to the +1 subsite in other glycosidases (Fig. 4B). The side-chain orientation of Tyr181 is, however, different in the EGCII aglycon site compared with Tyr224 and Phe169 in GBA1 and GBA3, respectively. The apparent conservation of some aromatic amino acid residues in the aglycon binding site suggests an important role in the positioning of glycosphingolipid substrate. The commonality in the overall architecture of the substrate binding sites among different glycosylceramidases might explain the paradox of different fold and low primary structure similarity on the one hand and joint substrate specificities on the other hand.
Fig. 4.
Glycosylceramidases: binding site architecture and stabilization. A: Superposition of the glycon binding sites of EGC II [PDB ID: 2osx (37); blue], GBA [PDB ID: 2v3e (83); green], and GBA3 [PDB ID: 2e9m (66); magenta] in complex with d-glucose (ball-and-stick, blue), DNJ (ball-and-stick, green) and galactose (ball-and-stick, magenta), respectively. B: Superposition of the aglycon sites of EGC II in complex with glucosylceramide (ball-and-stick, blue), GBA1 in complex with NN-DNJ (ball-and-stick, green), and GBA3 (ball-and-stick, magenta). The conserved aromatic residues are shown in sticks. C: GBA1-rigidified regions upon IFG bonding depicted in red on the GBA1 crystal structure (PDB ID: 2v3e) as determined by HDX mass spectrometry (87). D: Tryptic cleavage site (Lys 194) highlighted in magenta spheres on the GBA1 structure (PDB ID: 1ogs) (33).
GLYCOSYLCERAMIDASES: DYNAMICS AND STABILITY
Glycosidases, like most enzymes, are dynamic entities that undergo multiple conformations during catalysis (84). Thus, one snapshot of protein structure is insufficient to completely understand the catalytic process of the enzyme. The molecular dynamics of retaining β-glycosidases have been studied little so far. The biophysical and theoretical techniques to study the global dynamics are molecular dynamic simulation, frequency decay fluorometry, hydrogen/deuterium exchange (HDX) mass spectrometry, and NMR spectroscopy, which covers a broad range of time-scale motion from picoseconds to hours with an atomic resolution.
GH5 and GH30 enzymes have a comparable catalytic TIM barrel catalytic domain. David et al. (85) determined the dynamics of this domain of β-(1,4)-glycosidase Cex (CfXyn10A) of the soil bacterium Cellulomonas fimi using NMR spectroscopy from the nanosecond to millisecond time scale. The TIM barrel domain was found to be uniformly rigid on the probed time scales. Few dynamic changes were observed for the glycosyl-enzyme intermediate-state complex, although an enhanced thermal stability and proteolysis resistance of the domain complexed with the 2-deoxy-2-fluoro-β-cellobioside was noted. A lower-resolution technique was also employed to follow the conformational dynamics of a thermophilic clan A β-glycosidase from the extremophilic archaeon S. solfataricus (Sβgly) and a mesophilic clan A β-glycosidase from Escherichia coli, using multitryptophan emission decay (86). The thermophilic Sβgly has 12 tryptophan residues mostly distributed over the core of the protein TIM barrel domain with some located on flexible loops. Two groups of tryptophan fluorescence decay were observed for Sβgly: a short-lived and a long-lived decay, reflecting the well-packed and rigid TIM barrel core and the flexibility of the protein surface loops, respectively. However, when incubated with the irreversible inhibitor cyclophellitol (see section below), quenching of the fluorescence of short-lived tryptophan residues occurred, pointing toward a structural rigidification of the TIM barrel’s flexible loops. In contrast, the mesophilic clan A β-glycosidase from E. coli showed a uniform distribution of tryptophan fluorescence decay, indicating a looser structure of its TIM barrel domain. The same trend was also observed for GH30 GBA1 when studied with HDX mass spectrometry. Residues residing in the core of the TIM barrel domain were highly protected against the HDX, and loops connecting the α-helices and β-sheet of the catalytic domain showed the lowest protection (87). The conformational stability and rigidity of GBA1 is pH-dependent, as indicated by measuring the melting temperature (Tm) at different pH values and resistance toward tryptic digestion (88). At acidic pH, similar to that of lysosomes, the Tm of GBA1 is 4°C higher than at neutral pH, as found in the ER. The enhanced GBA1 thermal stability under acidic conditions is accompanied by remarkable protection against digestion by trypsin. At neutral pH, GBA1 displays a major trypsin cleavage site located on a flexible loop in the proximity of the substrate binding site (Fig. 4D). Thus, a different local pH at distinct subcellular locations might influence the rigidity of GBA1 (88).
The amino acid substitutions in GBA1 that cause Gaucher disease provide information on structural requisites for the folding and stability of the enzyme. Mutations in the β-side structure, also named the folding domain, are generally found to impair folding in the ER and result in proteasomal degradation of the misfolded protein (89). The best-known example is the L444P substitution that results in low amounts (of catalytically normal) GBA1 in lysosomes (90). The L444P substitution occurs frequently in all ethnic groups, and homozygosity leads to severe neuronopathic variants of Gaucher disease. On the other hand, the N370S substitution in the GBA1 sequence, the most common mutation among Caucasians, results in (near) normal amounts of GBA1 in lysosomes (91). This mutant enzyme, however, shows abnormalities in catalytic parameters in vitro and in vivo (89, 92). Recombinant N370S GBA1 has been crystalized and studied with HDX mass spectrometry and crystallography (87, 93, 94). The crystal structures of mutant enzymes at acidic and neutral pH values showed a similar overall folding to that of normal enzymes. Subtle differences were noted in the conformation of a flexible loop at the active site and in the hydrogen bonding ability of aromatic residues on this loop with residue 370 and the catalytic residues Glu-235 and Glu-340. Circular dichroism spectroscopy showed a pH-dependent change in the environment of tryptophan residues in imiglucerase that is absent in N370S GBA1. This finding coincides with the earlier observation that N370S GBA1 shows a relatively high residual enzymatic activity at pH <5.0 (92). Again, the local pH in lysosomes might significantly influence features of mutant GBA1 and render an explanation for the variable severity of disease in Gaucher patients homozygous for N370S GBA1, ranging from prominent visceral symptoms to a virtual asymptomatic disease course (87).
In general, the TIM barrel domain of glycosidases seems globally rigid yet includes flexible loops. It is proposed that a rigid TIM barrel fold provides the correct frame for favorable interactions with carbohydrate substrates and that this rigidity is necessary to bind, distort, and subsequently hydrolyze the glycosidic linkage within the enzyme active site (85). The flexible loops may play essential roles in substrate binding and product release and influence the still poorly understood intermolecular interaction of GBA with its activator protein saposin C (95, 96).
No information on the dynamics of the (α/α)6 solenoid catalytic domain of GH116 family members is yet available. An investigation of TxGH116 provided information about the protein fold and led to tentative explanations for the detrimental effects of observed amino acid substitutions in human GBA2 that lead to neurological symptoms (57). For instance, it has been proposed that the substitution of Arg-360 by a tryptophan in GBA2 sequence leads to a local disturbance of the charge balance within the mutation vicinity and structural clashes due to the bulky tryptophan side chain, thus destabilizing the global fold of the protein. Another pathogenic mutation is F419V, which is located in the interface between the N-terminal β-sandwich and the (α/α)6 solenoid catalytic domains. Substitutions of the aromatic side chain by two methyl groups at this position is assumed to disrupt the interdomain interactions and induce a global destabilization of the protein fold.
TRANSLATION OF KNOWLEDGE: THERAPEUTIC AND INDUSTRIAL ENZYMES, THERAPEUTIC INHIBITORS, AND DIAGNOSTIC ACTIVITY-BASED PROBES
The interest of fundamental as well as applied nature in retaining β-glycosidases is presently high. Acquired fundamental knowledge on these enzymes increasingly finds a variety of important applications meriting discussion.
Therapeutic enzymes
Seminal investigations on GBA-deficient Gaucher disease patients receiving chronic intravenous administration of (recombinant) human GBA1 have led to the development of an effective treatment for visceral complications such as splenomegaly, hepatomegaly, low platelet counts, and anemia (97, 98). The success of this treatment, coined as enzyme replacement therapy, has prompted similar approaches for other inherited lysosomal disorders caused by the deficiency of a retaining glycosidase, such as Fabry disease (α-galactosidase A deficiency), Pompe disease (acid α-glucosidase deficiency), mucopolysaccharidosis I (α-iduronidase deficiency), and mucopolysaccharidosis VII (β-glucuronidase deficiency). Different production platforms (cultured transfected hamster cells, gene-activated human cells, and genetically modified rabbits and plant cells) are presently in use to produce therapeutic glycosidases for enzyme replacement therapies (98–101).
Examples of present intraintestinal use of glycosidases are recombinant lactase together with dairy products by individuals suffering from lactose intolerance and recombinant α-galactosidase to prevent flatulence following the consumption of galactomannan-rich plant food (102, 103).
Therapeutic noncovalent inhibitors
Inhibitors of retaining β-glycosidases have received considerable attention. They provide important research tools and can now be found in therapeutic applications. Two fundamentally different classes of inhibitors can be distinguished: reversible noncovalent inhibitors and irreversible covalent inhibitors. Numerous reversible β-glycosidase inhibitors, including saccharides, iminosugars, carbasugars, thiosugars, azosugars, and nonglycosidic inhibitors, have either been synthesized or extracted from natural sources (104). Reversible β-glycosidase inhibitors are considered attractive for medical use because the inhibition of target (or off-target) enzymes can be more easily controlled. Intestinal α-glycosidase inhibitors, which were developed for the oral treatment of type 2 diabetes, were among the first applied inhibitors in the clinic. Inside the intestine these inhibitors reduce the conversion of complex carbohydrates, such as starch, to monosaccharides, which are absorbed by the body. Thus, α-glucosidase inhibitors reduce the risk of postprandial hyperglycemia (105). Examples of α-glucosidase inhibitors in clinical use are acarbose, voglibose, and the iminosugar miglitol (N-hydroxyethyldeoxynojirimycin) (106).
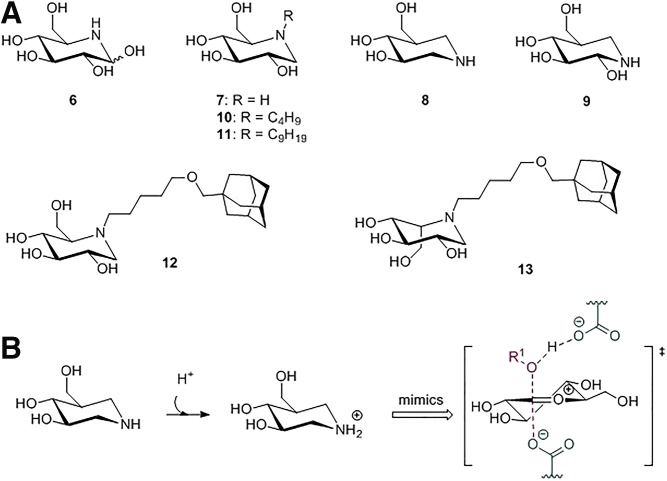
Iminosugars that modulate glycosidases deserve special discussion. They are presently most actively studied as therapeutic agents in patients with lysosomal glycosidase deficiencies. In particular, their beneficial action as pharmacological (chemical) chaperones that enhance the folding and/or stability of mutant glycosidases is being pursued (see below) (107–109). Iminosugars (Fig. 5A) are small polyhydroxylated alkaloids with at least two hydroxyl groups and one heterocyclic nitrogen atom. Simple iminosugars naturally occur in plants and microorganisms (110). The substitution of the oxygen or anomeric carbon of the pyranose ring by protonated nitrogen is thought to mimic the positive charge generated in these centers upon partial cleavage of the glycosidic bond, thus emulating the oxocarbenium ion transition state with a high inhibitory potency (111) (Fig. 5B). Nojirimycin (NJ) (compound 6; Fig. 5A), isolated from Bacillus, Streptomyces, and mulberry, was shown to be a potent inhibitor of α- and β-glycosidases (104). The presence of a hydroxyl group at C1 renders NJ unstable, hampering exploitation. 1-Deoxynojirimycin (DNJ) (compound 7; Fig. 5A) lacks the C1 hydroxyl that enhances stability and inhibitory potency (104). DNJ also inhibits α- and β-glycosidases. Changing the position of the heterocycle nitrogen atom to the anomeric carbon position of DNJ pyranosidic ring in so-called 1-azasugars increases the inhibition of β-glucosidases 440 times without affecting α-glycosidases (112). Isofagomine (IFG) (compound 8; Fig. 5A) is a 1-azasugar in which the anomeric carbon and the ring oxygen of glucose are replaced by nitrogen and carbon, respectively, and the C2 hydroxylic group is absent. The remaining hydroxyl groups are maintained, preserving a d-glucose-like configuration. In noeuromycin (compound 9), the presence of the hydroxyl group at C2 increases β-glycosidase inhibition, presumably due to an additional hydrogen bond interaction in the active site (113).
Fig. 5.
Iminosugar inhibitors. A: Chemical structures: NJ (6), DNJ (7), IFJ (8), noeuromycin (9), NB-DNJ (10), NN-DNJ (11), AMP-DNM (12), and l-idose-configured iminosugar substituted with methoxypentyl-adamantyl (13). B: IFG is protonated by the acid/base amino acid and mimics the positive charge of the oxocarbenium ion transition state.
The high specificity of IFG toward GBA1 has prompted studies on its use as a therapeutic chemical chaperone in Gaucher disease patients. These patients produce mutant GBA1 molecules that often are inefficiently folded in the ER and/or show reduced stability in lysosomes (17). IFG shows a higher binding affinity to GBA1 at neutral pH (as in the ER) compared with acidic pH (as in lysosomes) (56). IFG was envisioned to assist the folding and/or stability of (mutant) GBA1 and, because it crosses the blood-brain barrier, to have the potential to improve neuropathology in severely affected Gaucher disease patients. Positive effects of IFG on (mutant) GBA1 were noted in in vitro and in vivo studies (114). IFG was found to increase the thermal stability of GBA and to increase in cultured cells the levels of wild-type N370S and L444P GBA, the two most commonly encountered mutant enzymes in Gaucher disease patients (115). Unfortunately, IFG has failed in clinical trials, likely due to difficulties in reaching optimal IFG concentrations concomitantly in several tissues. Of note, various glucomimetics are now under development, and their effects on chaperone capacity are being investigated (109, 116). Using HDX mass spectrometry, strong perturbations in the HDX rate were observed for regions in GBA engaged in direct interaction with IFG as well as in other distal protein regions. In contrast, in the crystalline state no significant structural changes were observed in GBA1 upon IFG binding (117). The IFG rigidification effects on GBA1 seem to propagate beyond its binding pocket to surrounding regions, thus restricting the local protein dynamics. Recently, the occupancy effect of the binding site in GBA1 by small hydrophilic and amphiphilic mechanism-based inhibitors was studied (88). The complex of GBA1 with the small glycomimetic conduritol B epoxide (CBE) was found to cause only minor changes in the enzyme fluorescence spectrum. In contrast, binding of activity-based probes with a large amphiphilic extension occupying both glycon and aglycon binding sites led to a prominent shift of the maximum GBA1 fluorescence spectra toward the blue region. This indicates an induced closed and rigid structure, which is also suggested by accompanying increased resistance to tryptic digestion (Fig. 3D). Thus, dual occupancy of the glycon and aglycon binding sites might enhance stabilization. Similar findings were made for EGCII, suggesting that amphiphilic compounds act as a “hydrophobic zipper” (118).
NB-DNJ (miglustat) (compound 10; Fig. 5A) is registered as therapeutic agent for the treatment of mild-to-moderate Gaucher disease by substrate reduction therapy (119–121). At micromolar concentrations NB-DNJ inhibits the enzyme glucosylceramide synthase (GCS) that generates glucosylceramide from ceramide and UDP glucose (122). NB-DNJ is thus able to reduce glucosylceramide and glycosphingolipids (105). More recently, NB-DNJ was also found to stabilize GBA1 (123). NN-DNJ (compound 11; Fig. 5A) shows even considerably better binding affinity toward GBA1, presumably due to its longer alkyl chain length (124). Another target of N-substituted deoxynojirimycin is GBA2 (125). This β-glucosidase is very sensitive to inhibition by hydrophobic deoxynojirimycins such as N-(5)-adamantane-1-yl-methoxy-pentyl deoxynojirimycin (AMP-DNM) (compound 12; Fig. 5A) (IC50 = 3 nM) (126). The inhibition of GBA2 has been found to be beneficial in GBA1-deficient mice and animals with other deficiencies in lysosomal glycosphingolipid degradation (127, 128). Likewise, the genetic loss of GBA2 in these models was found to ameliorate disease, again suggesting that excessive metabolism of glucosylceramide by GBA2 during impaired lysosomal degradation contributes to disease severity (128, 129).
The tuning of the N-substituent of deoxynojirimycins could render exciting therapeutic agents (85). An extensive library of N-substituted deoxynojirimycins has been synthesized and tested for its ability to inhibit key enzymes in the metabolism of glucosylceramide: GBA1, GBA2, and GCS (125, 130, 131). Deoxynojirimycins were substituted with a butyl, methoxypentyl-adamantyl, or biphenyl moiety. Desired for the treatment of Gaucher disease and other sphingolipid storage disorders are compounds that concomitantly inhibit GCS and GBA2 with high affinity but do not impair GBA1 enzymatic activity. Optimal in this respect is l-idose-configured iminosugar substituted with methoxypentyl-adamantyl (compound 13; Fig. 5A) (132). d-Glucose-configured AMP-DNM also exerts major beneficial effects in obese rodents such as improving glucose homeostasis, correcting hepatosteatosis, and restoring satiety (133–136). The remarkable positive effect of AMP-DNM on glucose homeostasis could be attributed to the combined buffering of carbohydrate assimilation through the inhibition of intestinal glycosidases and tissue-wide improvement of insulin sensitivity through the lowering of inhibitory gangliosides in lipid rafts on the surface of cells (131).
Covalent mechanism-based inhibitors and diagnostic activity-based probes
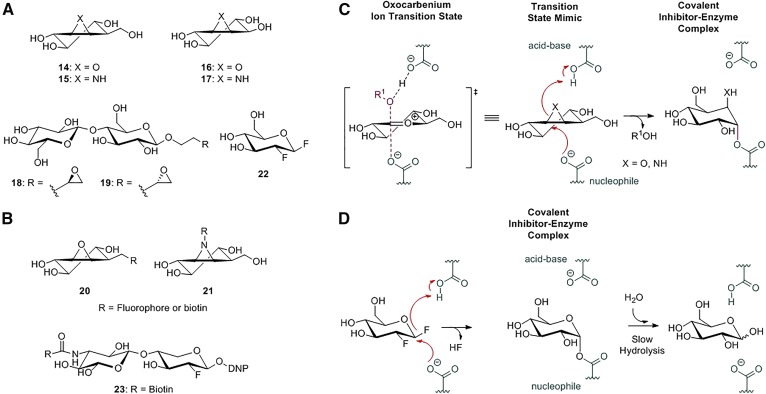
Cyclophellitol (compound 14; Fig. 6A), extracted from the mushroom Phellinus sp., was shown to be a potent suicide inhibitor of retaining β-glucosidases such as GBA1 (137). Cyclophellitol can induce GBA1 deficiency in cells and rodents (138, 139). It does this by binding covalently, in a mechanism-based manner, to the catalytic nucleophile E340 of the enzyme (Fig. 6B) (140, 141). Cyclophellitol aziridine (compound 15; Fig. 6A) also acts as a suicide inhibitor via the same mechanism. The inactivation involves the nucleophilic attack by the enzyme’s catalytic nucleophile to the cyclophellitol, or cyclophellitol aziridine electrophile, resulting in ring opening and the formation of a covalent adduct bond to the nucleophile. The reaction is facilitated by protonation of the cyclophellitol by the general acid/base residue (Fig. 6B). Cyclophellitol aziridine exhibits a positive charge and is intrinsically more reactive than the epoxide counterpart (142).
Fig. 6.
Retaining β-glycosidase mechanism-based covalent inhibitors and ABPs. A: Chemical structures of epoxide-, aziridine-, and fluorinated-based inactivators: cyclophellitol (14), cyclophellitol aziridine (15), conduritol B (16), conduritol aziridine (17), exo-alkyl epoxide glycosides (18 and 19), and 2-deoxy-2-fluoro glucoside (22). B: ABPs targeting glycosidases: cyclophellitol (aziridine) ABPs (20 and 21) and 2-deoxy-2-fluoro glycoside-based ABP (23). C: Cyclophellitol inactivation mechanism: cyclophellitol analogues irreversibly inhibit glycosidases by mimicking the oxocarbenium ion transition state and employing epoxides or aziridines as an electrophilic trap. D: 2-Deoxy-2-fluoro glucoside inactivators irreversibly inhibit glycosidases, forming a covalent inhibitor-enzyme complex that is subsequently slowly hydrolyzed.
CBE (compound 16; Fig. 6A) employs the same mechanism for the irreversible inactivation of glycosidases (140). It was used to identify the catalytic carboxylate in sucrase and isomaltase (143). Radioactive CBE derivatives were also employed to identify catalytic active-site residues in other glycosidases (144). The structural symmetry of CBE allows the labeling of both α- and β-glucosidases (145, 146). However, CBE may interact not only with catalytic nucleophile residues. For instance, the assignment of nucleophile residues of β-lac Z, GBA1, and almond β-glucosidase nucleophile residues by CBE was first erroneous and later corrected (147, 148). The aziridine analogue of CBE (compound 17; Fig. 6A) is a modest inactivator of Abg β-glycosidase and more potently of yeast α-glucosidase (149). Exo-alkyl epoxide glycosides (compounds 18 and 19; Fig. 6A), with an epoxide moiety linked to a monosaccharide by an alkyl chain, were also used to label the catalytic nucleophile in a variety of enzymes, and through X-ray crystallography their interactions with the enzymes were characterized (150). It was observed that the alkyl chain length may influence the labeling. In a retaining 1,4 xylanase from Trichoderma reesi, 2,3-epoxypropyl β-d-xyloside and 3,4-epoxybutyl β-d-xyloside labeled the nucleophile and acid base residue, respectively (151).
CBE has meanwhile found many research applications. Administering CBE to mice has rendered insight in the minimum GBA activity (12% to 16%) required for normal functioning (152). CBE inactivates GBA1 in all tissues, including the brain. Therefore, CBE treatment of mice allows investigations on CNS pathology resulting from GBA deficiency (153). The cyclitol was also used to study the importance of glucosylceramide metabolism in cancer cells resistant to chemotherapy-induced apoptosis (154, 155). It furthermore assisted the discovery of the occurrence of the second cellular glucosylceramidase, GBA2 (20).
Covalent inhibitors such as cyclophellitol have provided insights on intermediate-state sugar ring distortions. The crystal structure of cyclophellitol covalently bound in β-glucosidase from Thermotoga maritima, a bacterial β-glucosidase, revealed a 4C1 chair conformation (156). Witte et al. (157) designed selective activity-based probes (ABPs) toward GBA1 based on cyclophellitol as a scaffold (ABP 20; Fig. 6B). A reporter group (biotin or BODIPY) was attached to cyclophellitol via a pentyl linker. The resulting ABP allows ultrasensitive and specific visualization of GBA1 in vitro as well as in cultured cells and mice (157). Subsequently designed ABPs were based on a cyclophellitol aziridine scaffold with attached reporter groups via alkyl or acyl linkers (ABP 21; Fig. 6B) (158). These probes generally recognize multiple retaining glycosidases in the same class (159). Meanwhile, cyclophellitol aziridine ABPs labeling α-galactosidases, α-glucosidases, α-fucosidase, β-galactosidases, and β-glucuronidase have been successfully generated (160–164). These ABPs find several applications, such as quantitative detection and localization of glycosidases in cells and tissues, as well as identification and characterization of glycosidase inhibitors by competitive ABP profiling (165–167). Another important application lies in the demonstration of the deficiency of glycosidases in assisting the diagnosis of corresponding inherited diseases. The remarkable sensitivity of glycosidase detection with fluorescent ABPs allows the use of small amounts of analytes such as cells and bodily fluids (78).
Another class of covalent retaining glycosidase inhibitors are 2-deoxy-2-fluoro glycosides (compound 22; Fig. 6A). These substrate mimics contain an excellent leaving group (a fluoride) linked to a glycoside at C2. This C2 fluoride stabilizes the glycosyl-enzyme intermediate (Fig. 6D) (168). The spontaneous hydrolysis of the glycosyl-enzyme complex is slow; the half-life may vary between seconds to hours. The enzyme’s catalytic activity can be rescued with a suitable external acceptor to transfer the fluorinated glycoside and liberate the nucleophile residue (168). This feature has been used to explore the aglycon binding site. The recovery of enzyme activity complexed with fluorinated glycosides relies on the binding affinity of an acceptor to the aglycon site (169). Like CBE and cyclophellitol, activated fluorinated glycosides have been amply used as research tools for studying retaining β-glycosidases (170). NMR spectrometry with 2-deoxy-2-fluoro glycoside provided a model of substrate itinerary and insight in enzyme dynamics during catalysis in β-glucosidase from Alcaligenes faecalis and β-(1,4)-glycosidase from C. fimi, respectively (85, 171). Fluorinated glycosides assisted the identification of a novel mammalian cytosolic β-glucosidase and characterization of the catalytic pockets of lactase phlorizin hydrolase (172, 173). In addition, 2-deoxy-2-fluoro glycosides were also developed into ABPs (174). These were able to label β-glycosidases selectively in complex biological mixtures and assisted in the discovery of novel β-glycosidase enzymes in C. fimi (ABP 23; Fig. 6B, D) (175).
CONCLUSION AND PERSPECTIVE
Knowledge on retaining β-glucosidases has rapidly expanded over the last few decades. Structure activity relationship and dynamics studies provide detailed information about their physical characteristics and deepen the understanding of substrate-to-product itineraries. This approach has led to more potent and selective inhibitors and chemical chaperones, although their full potential in therapeutic applications has yet to be realized. Understanding the role of protein dynamics in substrate binding and distortion and in product release is comparatively small. Further information on this aspect could solve the paradox between relatively high structural conservation and a wide variety of functions among some glycosidases.
This review focused on glycosylceramidases. This field in particular has witnessed a remarkable successful translation of fundamental knowledge to clinical care. Now in use as registered drugs are recombinant glycosidases as well as modulators of glucosylceramide metabolism. Furthermore, the therapeutic value of chemical chaperones in enhancing mutant glycosidases is actively being investigated. ABPs now facilitate diagnoses of enzyme deficiency and the screening for agents that interact with the catalytic pocket of glycosidases.
Footnotes
Abbreviations:
- ABP
- activity-based probe
- AMP-DNM
- N-(5)-adamantane-1-yl-methoxy-pentyl
- CBE
- conduritol B epoxide
- DNJ
- 1-deoxynojirimycin
- EGCI
- endoglycoceramidase I
- EGCII
- endoglycoceramidase II
- GALC
- galactocerebrosidase
- GBA1
- glucocerebrosidase
- GBA2
- glucosylceramidase
- GCS
- glucosylceramide synthase
- GH
- glycoside hydrolase
- GlcCer
- β-glucosylceramide
- HDX
- hydrogen/deuterium exchange
- IFG
- isofagomine
- PDB
- Protein Data Bank
- TxGH116
- GH116 β-glucosidase of Thermoanaerobacterium xylanolyticum
- LPH
- lactase phlorizin hydrolase
- M6P
- mannose-6-phosphate
- NB-DNJ
- N-butyldeoxynojirimycin
- NJ
- nojirimycin
- NN-DNJ
- N-nonyldeoxynojirimycin
- Sβgly
- clan A β-glycosidase from Sulfolobus solfataricus
This work was supported by European Research Council Grant AdvG-290836 CHEMBIOSPHING, NRSCB NWO (H.S.O.), an NWO-CW ChemThem grant (H.S.O., J.M.F.G.A.), ZonMW Toppunt (H.S.O., J.M.F.G.A.), and Sanofi Genzyme (research grant to H.S.O. and J.M.F.G.A. and postdoctoral contract to M.A.).
REFERENCES
- 1.Laine R. A. 1994. A calculation of all possible oligosaccharide isomers both branched and linear yields 1.05x10(12) structures for a reducing hexasaccharide—the isomer-barrier to development of single-method saccharide sequencing or synthesis systems. Glycobiology. 4: 759–767. [DOI] [PubMed] [Google Scholar]
- 2.Wolfenden R., Lu X. D., and Young G.. 1998. Spontaneous hydrolysis of glycosides. J. Am. Chem. Soc. 120: 6814–6815. [Google Scholar]
- 3.Davies G. J., Gloster T. M., and Henrissat B.. 2005. Recent structural insights into the expanding world of carbohydrate-active enzymes. Curr. Opin. Struct. Biol. 15: 637–645. [DOI] [PubMed] [Google Scholar]
- 4.Garg S. 2016. Xylanase: applications in biofuel production. Curr. Metabolomics. 4: 23–37. [Google Scholar]
- 5.Collins T., Hoyoux A., Dutron A., Georis J., Genot B., Dauvrin T., Arnaut F., Gerday C., and Feller G.. 2006. Use of glycoside hydrolase family 8 xylanases in baking. J. Cereal Sci. 43: 79–84. [Google Scholar]
- 6.Asano N., Nash R. J., Molyneux R. J., and Fleet G. W. J.. 2000. Sugar-mimic glycosidase inhibitors: natural occurrence, biological activity and prospects for therapeutic application. Tetrahedron Asymmetry. 11: 1645–1680. [Google Scholar]
- 7.von Itzstein M., and Colman P.. 1996. Design and synthesis of carbohydrate-based inhibitors of protein-carbohydrate interactions. Curr. Opin. Struct. Biol. 6: 703–709. [DOI] [PubMed] [Google Scholar]
- 8.Asano N. 2003. Glycosidase inhibitors: update and perspectives on practical use. Glycobiology. 13: 93R–104R. [DOI] [PubMed] [Google Scholar]
- 9.Neufeld E. F. 1991. Lysosomal storage diseases. Annu. Rev. Biochem. 60: 257–280. [DOI] [PubMed] [Google Scholar]
- 10.Sibley E. 2004. Carbohydrate intolerance. Curr. Opin. Gastroenterol. 20: 162–167. [DOI] [PubMed] [Google Scholar]
- 11.Davies G. J., and Williams S. J.. 2016. Carbohydrate-active enzymes: sequences, shapes, contortions and cells. Biochem. Soc. Trans. 44: 79–87. [DOI] [PubMed] [Google Scholar]
- 12.Henrissat B. 1991. A classification of glycosyl hydrolases based on amino-acid-sequence similarities. Biochem. J. 280: 309–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Henrissat B., and Davies G.. 1997. Structural and sequence-based classification of glycoside hydrolases. Curr. Opin. Struct. Biol. 7: 637–644. [DOI] [PubMed] [Google Scholar]
- 14.Aerts J. M., Hollak C., Boot R., and Groener A.. 2003. Biochemistry of glycosphingolipid storage disorders: implications for therapeutic intervention. Philos. Trans. R. Soc. Lond. B Biol. Sci. 358: 905–914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gaucher P. C. E. 1882. De l’épithélioma primitif de la rate. Hypertrophie idiopathique de la rate sans leucémie. PhD Dissertation. University of Paris, Paris, France. French. [Google Scholar]
- 16.Brady R. O., Kanfer J., Bradley R., and Shapiro D.. 1966. Demonstration of a deficiency of glucocerebroside-cleaving enzyme in Gaucher’s disease. J. Clin. Invest. 45: 1112–1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zimmer K. P., le Coutre P., Aerts H. M., Harzer K., Fukuda M., O’Brien J. S., and Naim H. Y.. 1999. Intracellular transport of acid β-glucosidase and lysosome-associated membrane proteins is affected in Gaucher’s disease (G202R mutation). J. Pathol. 188: 407–414. [DOI] [PubMed] [Google Scholar]
- 18.Sidransky E., Nalls M. A., Aasly J. O., Aharon-Peretz J., Annesi G., Barbosa E. R., Bar-Shira A., Berg D., Bras J., and Brice A.. 2009. Multicenter analysis of glucocerebrosidase mutations in Parkinson’s disease. N. Engl. J. Med. 361: 1651–1661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Siebert M., Sidransky E., and Westbroek W.. 2014. Glucocerebrosidase is shaking up the synucleinopathies. Brain. 137: 1304–1322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.van Weely S., Brandsma M., Strijland A., Tager J. M., and Aerts J. M.. 1993. Demonstration of the existence of a second, non-lysosomal glucocerebrosidase that is not deficient in Gaucher disease. Biochim. Biophys. Acta. 1181: 55–62. [DOI] [PubMed] [Google Scholar]
- 21.Hammer M. B., Eleuch-Fayache G., Schottlaender L. V., Nehdi H., Gibbs J. R., Arepalli S. K., Chong S. B., Hernandez D. G., Sailer A., and Liu G.. 2013. Mutations in GBA2 cause autosomal-recessive cerebellar ataxia with spasticity. Am. J. Hum. Genet. 92: 245–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Martin E., Schüle R., Smets K., Rastetter A., Boukhris A., Loureiro J. L., Gonzalez M. A., Mundwiller E., Deconinck T., and Wessner M.. 2013. Loss of function of glucocerebrosidase GBA2 is responsible for motor neuron defects in hereditary spastic paraplegia. Am. J. Hum. Genet. 92: 238–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kancheva D., Atkinson D., De Rijk P., Zimon M., Chamova T., Mitev V., Yaramis A., Fabrizi G. M., Topaloglu H., and Tournev I.. 2016. Novel mutations in genes causing hereditary spastic paraplegia and Charcot-Marie-Tooth neuropathy identified by an optimized protocol for homozygosity mapping based on whole-exome sequencing. Genet. Med. 18: 600–607. [Erratum. 2016. Genet. Med. 18: 108. ] [DOI] [PubMed] [Google Scholar]
- 24.Sultana S., Reichbauer J., Schüle R., Mochel F., Synofzik M., and van der Spoel A. C.. 2015. Lack of enzyme activity in GBA2 mutants associated with hereditary spastic paraplegia/cerebellar ataxia (SPG46). Biochem. Biophys. Res. Commun. 465: 35–40. [DOI] [PubMed] [Google Scholar]
- 25.Rye C. S., and Withers S. G.. 2000. Glycosidase mechanisms. Curr. Opin. Chem. Biol. 4: 573–580. [DOI] [PubMed] [Google Scholar]
- 26.Speciale G., Thompson A. J., Davies G. J., and Williams S. J.. 2014. Dissecting conformational contributions to glycosidase catalysis and inhibition. Curr. Opin. Struct. Biol. 28: 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ardèvol A., and Rovira C.. 2015. Reaction mechanisms in carbohydrate-active enzymes: glycoside hydrolases and glycosyltransferases. Insights from ab initio quantum mechanics/molecular mechanics dynamic simulations. J. Am. Chem. Soc. 137: 7528–7547. [DOI] [PubMed] [Google Scholar]
- 28.Danby P. M., and Withers S. G.. 2016. Advances in enzymatic glycoside synthesis. ACS Chem. Biol. 11: 1784–1794. [DOI] [PubMed] [Google Scholar]
- 29.Ribeirão M., Pereira-Chioccola V. L., Eichinger D., Rodrigues M. M., and Schenkman S.. 1997. Temperature differences for trans-glycosylation and hydrolysis reaction reveal an acceptor binding site in the catalytic mechanism of Trypanosoma cruzi trans-sialidase. Glycobiology. 7: 1237–1246. [DOI] [PubMed] [Google Scholar]
- 30.Eneyskaya E. V., Brumer H., Backinowsky L. V., Ivanen D. R., Kulminskaya A. A., Shabalin K. A., and Neustroev K. N.. 2003. Enzymatic synthesis of β-xylanase substrates: transglycosylation reactions of the β-xylosidase from Aspergillus sp. Carbohydr. Res. 338: 313–325. [DOI] [PubMed] [Google Scholar]
- 31.Akaike E., Tsutsumida M., Osumi K., Fujita M., Yamanoi T., Yamamoto K., and Fujita K.. 2004. High efficiency of transferring a native sugar chain from a glycopeptide by a microbial endoglycosidase in organic solvents. Carbohydr. Res. 339: 719–722. [DOI] [PubMed] [Google Scholar]
- 32.Davies G., and Henrissat B.. 1995. Structures and mechanisms of glycosyl hydrolases. Structure. 3: 853–859. [DOI] [PubMed] [Google Scholar]
- 33.St John F. J., Gonzalez J. M., and Pozharski E.. 2010. Consolidation of glycosyl hydrolase family 30: a dual domain 4/7 hydrolase family consisting of two structurally distinct groups. FEBS Lett. 584: 4435–4441. [DOI] [PubMed] [Google Scholar]
- 34.Aspeborg H., Coutinho P. M., Wang Y., Brumer H., and Henrissat B.. 2012. Evolution, substrate specificity and subfamily classification of glycoside hydrolase family 5 (GH5). BMC Evol. Biol. 12: 186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Durand P., Lehn P., Callebaut I., Fabrega S., Henrissat B., and Mornon J-P.. 1997. Active-site motifs of lysosomal acid hydrolases: invariant features of clan GH-A glycosyl hydrolases deduced from hydrophobic cluster analysis. Glycobiology. 7: 277–284. [DOI] [PubMed] [Google Scholar]
- 36.Ito M., and Yamagata T.. 1986. A novel glycosphingolipid-degrading enzyme cleaves the linkage between the oligosaccharide and ceramide of neutral and acidic glycosphingolipids. J. Biol. Chem. 261: 14278–14282. [PubMed] [Google Scholar]
- 37.Caines M. E., Vaughan M. D., Tarling C. A., Hancock S. M., Warren R. A. J., Withers S. G., and Strynadka N. C.. 2007. Structural and mechanistic analyses of endo-glycoceramidase II, a membrane-associated family 5 glycosidase in the Apo and GM3 ganglioside-bound forms. J. Biol. Chem. 282: 14300–14308. [DOI] [PubMed] [Google Scholar]
- 38.Han Y-B., Chen L-Q., Li Z., Tan Y-M., Feng Y., and Yang G-Y.. 2017. Structural insights into the broad substrate specificity of a novel endoglycoceramidase I belonging to a new subfamily of GH5 glycosidases. J. Biol. Chem. 292: 4789–4800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Li S-C., Degasperi R., Muldrey J. E., and Li Y-T.. 1986. A unique glycosphingolipid-splitting enzyme (ceramide-glycanase from leech) cleaves the linkage between the oligosaccharide and the ceramide. Biochem. Biophys. Res. Commun. 141: 346–352. [DOI] [PubMed] [Google Scholar]
- 40.Zhou B., Li S., Laine R. A., Huang R., and Li Y.. 1989. Isolation and characterization of ceramide glycanase from the leech, Macrobdella decora. J. Biol. Chem. 264: 12272–12277. [PubMed] [Google Scholar]
- 41.Carter B. Z., Li S-C., and Li Y-T.. 1992. Ceramide glycanase from the earthworm, Lumbricus terrestris. Biochem. J. 285: 619–623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Basu S. S., Dastgheibhosseini S., Hoover G., Li Z., and Basu S.. 1994. Analysis of glycosphingolipids by fluorophore-assisted carbohydrate electrophoresis using ceramide glycanase from Mercenaria mercenaria. Anal. Biochem. 222: 270–274. [DOI] [PubMed] [Google Scholar]
- 43.Pavlova N. V., Li S-C., and Li Y-T.. 2018. Degradation of glycosphingolipids in oyster: ceramide glycanase and ceramidase in the hepatopancreas of oyster, Crassostrea virginica. Glycoconj. J. 35: 77–86. [DOI] [PubMed] [Google Scholar]
- 44.Basu M., Kelly P., O’donnell P., Miguel M., Bradley M., Sonnino S., Banerjee S., and Basu S.. 1999. Ceramide glycanase activities in human cancer cells. Biosci. Rep. 19: 449–460. [DOI] [PubMed] [Google Scholar]
- 45.Basu M., Kelly P., Girzadas M., Li Z., and Basu S.. 2000. Properties of animal ceramide glycanases. Methods Enzymol. 311: 287–297. [DOI] [PubMed] [Google Scholar]
- 46.Akiyama H., Kobayashi S., Hirabayashi Y., and Murakami-Murofushi K.. 2013. Cholesterol glucosylation is catalyzed by transglucosylation reaction of β-glucosidase 1. Biochem. Biophys. Res. Commun. 441: 838–843. [DOI] [PubMed] [Google Scholar]
- 47.Marques A. R., Mirzaian M., Akiyama H., Wisse P., Ferraz M. J., Gaspar P., Ghauharali-van der Vlugt K., Meijer R., Giraldo P., and Alfonso P.. 2016. Glucosylated cholesterol in mammalian cells and tissues: formation and degradation by multiple cellular β-glucosidases. J. Lipid Res. 57: 451–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Vaughan M. D., Johnson K., DeFrees S., Tang X., Warren R. A. J., and Withers S. G.. 2006. Glycosynthase-mediated synthesis of glycosphingolipids. J. Am. Chem. Soc. 128: 6300–6301. [DOI] [PubMed] [Google Scholar]
- 49.Rich J. R., Cunningham A-M., Gilbert M., and Withers S. G.. 2011. Glycosphingolipid synthesis employing a combination of recombinant glycosyltransferases and an endoglycoceramidase glycosynthase. Chem. Commun. (Camb.). 47: 10806–10808. [DOI] [PubMed] [Google Scholar]
- 50.Ito M., and Yamagata T.. 1989. Purification and characterization of glycosphingolipid-specific endoglycosidases (endoglycoceramidases) from a mutant strain of Rhodococcus sp. Evidence for three molecular species of endoglycoceramidase with different specificities. J. Biol. Chem. 264: 9510–9519. [PubMed] [Google Scholar]
- 51.Ishibashi Y., Nakasone T., Kiyohara M., Horibata Y., Sakaguchi K., Hijikata A., Ichinose S., Omori A., Yasui Y., and Imamura A.. 2007. A novel endoglycoceramidase hydrolyzes oligogalactosylceramides to produce galactooligosaccharides and ceramides. J. Biol. Chem. 282: 11386–11396. [DOI] [PubMed] [Google Scholar]
- 52.Ishibashi Y., Ikeda K., Sakaguchi K., Okino N., Taguchi R., and Ito M.. 2012. Quality control of fungus-specific glucosylceramide in Cryptococcus neoformans by endoglycoceramidase-related protein 1 (EGCrP1). J. Biol. Chem. 287: 368–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Watanabe T., Ito T., Goda H. M., Ishibashi Y., Miyamoto T., Ikeda K., Taguchi R., Okino N., and Ito M.. 2015. Sterylglucoside catabolism in Cryptococcus neoformans with endoglycoceramidase-related protein 2 (EGCrP2), the first steryl-β-glucosidase identified in fungi. J. Biol. Chem. 290: 1005–1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Watanabe T., Tani M., Ishibashi Y., Endo I., Okino N., and Ito M.. 2015. Ergosteryl-β-glucosidase (Egh1) involved in sterylglucoside catabolism and vacuole formation in Saccharomyces cerevisiae. Glycobiology. 25: 1079–1089. [DOI] [PubMed] [Google Scholar]
- 55.Cobucci-Ponzano B., Aurilia V., Riccio G., Henrissat B., Coutinho P. M., Strazzulli A., Padula A., Corsaro M. M., Pieretti G., and Pocsfalvi G.. 2010. A new archaeal β-glycosidase from sulfolobus solfataricus seeding a novel retaining β-glycan-specific glycoside hydrolase family along with the human non-lysosomal glucosylceramidase GBA2. J. Biol. Chem. 285: 20691–20703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kallemeijn W. W., Witte M. D., Voorn-Brouwer T. M., Walvoort M. T., Li K-Y., Codée J. D., van der Marel G. A., Boot R. G., Overkleeft H. S., and Aerts J. M.. 2014. A sensitive gel-based method combining distinct cyclophellitol-based probes for the identification of acid/base residues in human retaining β-glucosidases. J. Biol. Chem. 289: 35351–35362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Charoenwattanasatien R., Pengthaisong S., Breen I., Mutoh R., Sansenya S., Hua Y., Tankrathok A., Wu L., Songsiriritthigul C., and Tanaka H.. 2016. Bacterial β-glucosidase reveals the structural and functional basis of genetic defects in human glucocerebrosidase 2 (GBA2). ACS Chem. Biol. 11: 1891–1900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yildiz Y., Matern H., Thompson B., Allegood J. C., Warren R. L., Ramirez D. M., Hammer R. E., Hamra F. K., Matern S., and Russell D. W.. 2006. Mutation of β-glucosidase 2 causes glycolipid storage disease and impaired male fertility. J. Clin. Invest. 116: 2985–2994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Boot R. G., Verhoek M., Donker-Koopman W., Strijland A., van Marle J., Overkleeft H. S., Wennekes T., and Aerts J. M.. 2007. Identification of the non-lysosomal glucosylceramidase as β-glucosidase 2. J. Biol. Chem. 282: 1305–1312. [DOI] [PubMed] [Google Scholar]
- 60.Körschen H. G., Yildiz Y., Raju D. N., Schonauer S., Bönigk W., Jansen V., Kremmer E., Kaupp U. B., and Wachten D.. 2013. The non-lysosomal β-glucosidase GBA2 is a non-integral membrane-associated protein at the endoplasmic reticulum (ER) and Golgi. J. Biol. Chem. 288: 3381–3393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Walden C. M., Sandhoff R., Chuang C-C., Yildiz Y., Butters T. D., Dwek R. A., Platt F. M., and van der Spoel A. C.. 2007. Accumulation of glucosylceramide in murine testis, caused by inhibition of β-glucosidase 2: implications for spermatogenesis. J. Biol. Chem. 282: 32655–32664. [DOI] [PubMed] [Google Scholar]
- 62.Raju D., Schonauer S., Hamzeh H., Flynn K. C., Bradke F., vom Dorp K., Dörmann P., Yildiz Y., Trötschel C., and Poetsch A.. 2015. Accumulation of glucosylceramide in the absence of the beta-glucosidase GBA2 alters cytoskeletal dynamics. PLoS Genet. 11: e1005063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wacker H., Keller P., Falchetto R., Legler G., and Semenza G.. 1992. Location of the two catalytic sites in intestinal lactase-phlorizin hydrolase. Comparison with sucrase-isomaltase and with other glycosidases, the membrane anchor of lactase-phlorizin hydrolase. J. Biol. Chem. 267: 18744–18752. [PubMed] [Google Scholar]
- 64.Levin B., Abraham J., Burgess E. A., and Wallis P. G.. 1970. Congenital lactose malabsorption. Arch. Dis. Child. 45: 173–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Dekker N., Voorn-Brouwer T., Verhoek M., Wennekes T., Narayan R. S., Speijer D., Hollak C. E., Overkleeft H. S., Boot R. G., and Aerts J. M.. 2011. The cytosolic β-glucosidase GBA3 does not influence type 1 Gaucher disease manifestation. Blood Cells Mol. Dis. 46: 19–26. [DOI] [PubMed] [Google Scholar]
- 66.Hayashi Y., Okino N., Kakuta Y., Shikanai T., Tani M., Narimatsu H., and Ito M.. 2007. Klotho-related protein is a novel cytosolic neutral β-glycosylceramidase. J. Biol. Chem. 282: 30889–30900. [DOI] [PubMed] [Google Scholar]
- 67.Noguchi J., Hayashi Y., Baba Y., Okino N., Kimura M., Ito M., and Kakuta Y.. 2008. Crystal structure of the covalent intermediate of human cytosolic β-glucosidase. Biochem. Biophys. Res. Commun. 374: 549–552. [DOI] [PubMed] [Google Scholar]
- 68.Tribolo S., Berrin J-G., Kroon P. A., Czjzek M., and Juge N.. 2007. The crystal structure of human cytosolic β-glucosidase unravels the substrate aglycone specificity of a family 1 glycoside hydrolase. J. Mol. Biol. 370: 964–975. [DOI] [PubMed] [Google Scholar]
- 69.Cannizzaro L. A., Chen Y., Rafi M., and Wenger D. A.. 1994. Regional mapping of the human galactocerebrosidase gene (GALC) to 14q31 by in situ hybridization. Cytogenet. Cell Genet. 66: 244–245. [DOI] [PubMed] [Google Scholar]
- 70.Sakai N., Inui K., Midorikawa M., Okuno Y., Ueda S., Iwamatsu A., and Okada S.. 1994. Purification and characterization of galactocerebrosidase from human lymphocytes. J. Biochem. 116: 615–620. [DOI] [PubMed] [Google Scholar]
- 71.Deane J. E., Graham S. C., Kim N. N., Stein P. E., McNair R., Cachón-González M. B., Cox T. M., and Read R. J.. 2011. Insights into Krabbe disease from structures of galactocerebrosidase. Proc. Natl. Acad. Sci. USA. 108: 15169–15173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Harzer K., Paton B. C., Christomanou H., Chatelut M., Levade T., Hiraiwa M., and O’Brien J. S.. 1997. Saposins (sap) A and C activate the degradation of galactosylceramide in living cells. FEBS Lett. 417: 270–274. [DOI] [PubMed] [Google Scholar]
- 73.Hill C. H., Graham S. C., Read R. J., and Deane J. E.. 2013. Structural snapshots illustrate the catalytic cycle of β-galactocerebrosidase, the defective enzyme in Krabbe disease. Proc. Natl. Acad. Sci. USA. 110: 20479–20484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Tappino B., Biancheri R., Mort M., Regis S., Corsolini F., Rossi A., Stroppiano M., Lualdi S., Fiumara A., Bembi B., et al. . 2010. Identificationand characterization of 15 novel GALC gene mutations causing Krabbe disease. Hum. Mutat. 31: E1894–E1914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Suzuki K. 1998. Twenty five years of the “psychosine hypothesis”: a personal perspective of its history and present status. Neurochem. Res. 23: 251–259. [DOI] [PubMed] [Google Scholar]
- 76.Suzuki K. 1994. A genetic demyelinating disease globoid cell leukodystrophy: studies with animal models. J. Neuropathol. Exp. Neurol. 53: 359–363. [DOI] [PubMed] [Google Scholar]
- 77.Ferraz M. J., Marques A. R., Gaspar P., Mirzaian M., van Roomen C., Ottenhoff R., Alfonso P., Irún P., Giraldo P., and Wisse P.. 2016. Lyso-glycosphingolipid abnormalities in different murine models of lysosomal storage disorders. Mol. Genet. Metab. 117: 186–193. [DOI] [PubMed] [Google Scholar]
- 78.Ferraz M. J., Kallemeijn W. W., Mirzaian M., Moro D. H., Marques A., Wisse P., Boot R. G., Willems L. I., Overkleeft H., and Aerts J.. 2014. Gaucher disease and Fabry disease: new markers and insights in pathophysiology for two distinct glycosphingolipidoses. Biochim. Biophys. Acta. 1841: 811–825. [DOI] [PubMed] [Google Scholar]
- 79.Ferraz M. J., Marques A. R., Appelman M. D., Verhoek M., Strijland A., Mirzaian M., Scheij S., Ouairy C. M., Lahav D., and Wisse P.. 2016. Lysosomal glycosphingolipid catabolism by acid ceramidase: formation of glycosphingoid bases during deficiency of glycosidases. FEBS Lett. 590: 716–725. [DOI] [PubMed] [Google Scholar]
- 80.Sly W. S. 1985. Receptor-mediated transport of acid hydrolases to lysosomes. Curr. Top. Cell. Regul. 26: 27–38. [DOI] [PubMed] [Google Scholar]
- 81.Aerts J. M., Schram A., Strijland A., van Weely S., Jonsson L. M., Tager J. M., Sorrell S. H., Ginns E. I., Barranger J. A., and Murray G. J.. 1988. Glucocerebrosidase, a lysosomal enzyme that does not undergo oligosaccharide phosphorylation. Biochim. Biophys. Acta. 964: 303–308. [DOI] [PubMed] [Google Scholar]
- 82.Reczek D., Schwake M., Schröder J., Hughes H., Blanz J., Jin X., Brondyk W., Van Patten S., Edmunds T., and Saftig P.. 2007. LIMP-2 is a receptor for lysosomal mannose-6-phosphate-independent targeting of β-glucocerebrosidase. Cell. 131: 770–783. [DOI] [PubMed] [Google Scholar]
- 83.Brumshtein B., Greenblatt H. M., Butters T. D., Shaaltiel Y., Aviezer D., Silman I., Futerman A. H., and Sussman J. L.. 2007. Crystal structures of complexes of N-butyl-and N-nonyl-deoxynojirimycin bound to acid β-glucosidase: insights into the mechanism of chemical chaperone action in Gaucher disease. J. Biol. Chem. 282: 29052–29058. [DOI] [PubMed] [Google Scholar]
- 84.Henzler-Wildman K., and Kern D.. 2007. Dynamic personalities of proteins. Nature. 450: 964–972. [DOI] [PubMed] [Google Scholar]
- 85.Poon D. K. Y., Ludwiczek M. L., Schubert M., Kwan E. M., Withers S. G., and McIntosh L. P.. 2007. NMR spectroscopic characterization of a beta-(1,4)-glycosidase along its reaction pathway: stabilization upon formation of the glycosyl-enzyme intermediate. Biochemistry. 46: 1759–1770. [DOI] [PubMed] [Google Scholar]
- 86.Bismuto E., Nucci R., Rossi M., and Irace G.. 1999. Structural and dynamic aspects of beta-glycosidase from mesophilic and thermophilic bacteria by multitryptophanyl emission decay studies. Proteins. 35: 163–172. [PubMed] [Google Scholar]
- 87.Tang L., Coales S. J., Morrow J. A., Edmunds T., and Hamuro Y.. 2012. Characterization of the N370S mutant of glucocerebrosidase by hydrogen/deuterium exchange mass spectrometry. ChemBioChem. 13: 2243–2250. [DOI] [PubMed] [Google Scholar]
- 88.Ben Bdira F., Kallemeijn W. W., Oussoren S. V., Scheij S., Bleijlevens B., Florea B. I., van Roomen C. P., Ottenhoff R., van Kooten M. J., and Walvoort M. T.. 2017. Stabilization of glucocerebrosidase by active site occupancy. ACS Chem. Biol. 12: 1830–1841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Liou B., Kazimierczuk A., Zhang M., Scott C. R., Hegde R. S., and Grabowski G. A.. 2006. Analyses of variant acid β-glucosidases effects of Gaucher disease mutations. J. Biol. Chem. 281: 4242–4253. [DOI] [PubMed] [Google Scholar]
- 90.Ohashi T., Hong C., Weiler S., Tomich J., Aerts J., Tager J., and Barranger J.. 1991. Characterization of human glucocerebrosidase from different mutant alleles. J. Biol. Chem. 266: 3661–3667. [PubMed] [Google Scholar]
- 91.Jonsson L. M., Murray G. J., Sorrell S. H., Strijland A., Aerts J. F., Ginns E. I., Barranger J. A., Tager J. M., and Schram A. W.. 1987. Biosynthesis and maturation of glucocerebrosidase in Gaucher fibroblasts. Eur. J. Biochem. 164: 171–179. [DOI] [PubMed] [Google Scholar]
- 92.van Weely S., Van den Berg M., Barranger J., Miranda M. S., Tager J., and Aerts J.. 1993. Role of pH in determining the cell-type-specific residual activity of glucocerebrosidase in type 1 Gaucher disease. J. Clin. Invest. 91: 1167–1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Offman M. N., Krol M., Silman I., Sussman J. L., and Futerman A. H.. 2010. Molecular basis of reduced glucosylceramidase activity in the most common Gaucher disease mutant, N370S. J. Biol. Chem. 285: 42105–42114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Wei R. R., Hughes H., Boucher S., Bird J. J., Guziewicz N., Van Patten S. M., Qiu H., Pan C. Q., and Edmunds T.. 2011. X-ray and biochemical analysis of N370S mutant human acid β-glucosidase. J. Biol. Chem. 286: 299–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Yoneshige A., Muto M., Watanabe T., Hojo H., and Matsuda J.. 2015. The effects of chemically synthesized saposin C on glucosylceramide-β-glucosidase. Clin. Biochem. 48: 1177–1180. [DOI] [PubMed] [Google Scholar]
- 96.Gruschus J. M., Jiang Z., Yap T. L., Hill S. A., Grishaev A., Piszczek G., Sidransky E., and Lee J. C.. 2015. Dissociation of glucocerebrosidase dimer in solution by its co-factor, saposin C. Biochem. Biophys. Res. Commun. 457: 561–566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Brady R. O. 2003. Enzyme replacement therapy: conception, chaos and culmination. Philos. Trans. R. Soc. Lond. B Biol. Sci. 358: 915–919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Aerts J. M., and Cox T. M.. 2018. Roscoe O. Brady: physician whose pioneering discoveries in lipid biochemistry revolutionized treatment and understanding of lysosomal diseases. Blood Cells Mol. Dis. 68: 4–8. [DOI] [PubMed] [Google Scholar]
- 99.Parenti G., Andria G., and Ballabio A.. 2015. Lysosomal storage diseases: from pathophysiology to therapy. Annu. Rev. Med. 66: 471–486. [DOI] [PubMed] [Google Scholar]
- 100.Desnick R. J., and Schuchman E.. 2012. Enzyme replacement therapy for lysosomal diseases: lessons from 20 years of experience and remaining challenges. Annu. Rev. Genomics Hum. Genet. 13: 307–335. [DOI] [PubMed] [Google Scholar]
- 101.Zimran A., Brill-Almon E., Chertkoff R., Petakov M., Blanco-Favela F., Muñoz E. T., Solorio-Meza S. E., Amato D., Duran G., and Giona F.. 2011. Pivotal trial with plant cell-expressed recombinant glucocerebrosidase, taliglucerase alfa, a novel enzyme replacement therapy for Gaucher disease. Blood. 118: 5767–5773. [DOI] [PubMed] [Google Scholar]
- 102.Montalto M., Nucera G., Santoro L., and Curigliano V.. 2005. Effect of exogenous beta-galactosidase in patients with lactose malabsorption and intolerance: a crossover double-blind placebo-controlled study. Eur. J. Clin. Nutr. 59: 489–493. [DOI] [PubMed] [Google Scholar]
- 103.Di Stefano M., Miceli E., Gotti S., Missanelli A., Mazzocchi S., and Corazza G. R.. 2007. The effect of oral α-galactosidase on intestinal gas production and gas-related symptoms. Dig. Dis. Sci. 52: 78–83. [DOI] [PubMed] [Google Scholar]
- 104.de Melo E. B., da Silveira Gomes A., and Carvalho I.. 2006. α-and β-glucosidase inhibitors: chemical structure and biological activity. Tetrahedron. 62: 10277–10302. [Google Scholar]
- 105.Kennedy F. P., and Gerich J. E.. 1987. A new alpha-glucosidase inhibitor (Bay-m-1099) reduces insulin requirements with meals in insulin-dependent diabetes mellitus. Clin. Pharmacol. Ther. 42: 455–458. [DOI] [PubMed] [Google Scholar]
- 106.Van de Laar F. A., Lucassen P. L., Akkermans R. P., Van de Lisdonk E. H., Rutten G. E., and Van Weel C.. 2006. Alpha-glucosidase inhibitors for type 2 diabetes mellitus. Cochrane Libr. 18: CD005061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Boyd R. E., Lee G., Rybczynski P., Benjamin E. R., Khanna R., Wustman B. A., and Valenzano K. J.. 2013. Pharmacological chaperones as therapeutics for lysosomal storage diseases. J. Med. Chem. 56: 2705–2725. [DOI] [PubMed] [Google Scholar]
- 108.Fan J-Q. 2003. A contradictory treatment for lysosomal storage disorders: inhibitors enhance mutant enzyme activity. Trends Pharmacol. Sci. 24: 355–360. [DOI] [PubMed] [Google Scholar]
- 109.Benito J. M., García Fernández J. M., and Mellet C. O.. 2011. Pharmacological chaperone therapy for Gaucher disease: a patent review. Expert Opin. Ther. Pat. 21: 885–903. [DOI] [PubMed] [Google Scholar]
- 110.Watson A. A., Fleet G. W., Asano N., Molyneux R. J., and Nash R. J.. 2001. Polyhydroxylated alkaloids—natural occurrence and therapeutic applications. Phytochemistry. 56: 265–295. [DOI] [PubMed] [Google Scholar]
- 111.Sinnott M. L. 1990. Catalytic mechanism of enzymic glycosyl transfer. Chem. Rev. 90: 1171–1202. [Google Scholar]
- 112.Pandey G., Kapur M., Khan M. I., and Gaikwad S. M.. 2003. A new access to polyhydroxy piperidines of the azasugar class: synthesis and glycosidase inhibition studies. Org. Biomol. Chem. 1: 3321–3326. [DOI] [PubMed] [Google Scholar]
- 113.Liu H., Liang X., Søhoel H., Bülow A., and Bols M.. 2001. Noeuromycin, a glycosyl cation mimic that strongly inhibits glycosidases. J. Am. Chem. Soc. 123: 5116–5117. [DOI] [PubMed] [Google Scholar]
- 114.Khanna R., Benjamin E. R., Pellegrino L., Schilling A., Rigat B. A., Soska R., Nafar H., Ranes B. E., Feng J., and Lun Y.. 2010. The pharmacological chaperone isofagomine increases the activity of the Gaucher disease L444P mutant form of β-glucosidase. FEBS J. 277: 1618–1638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Sawkar A. R., Adamski-Werner S. L., Cheng W-C., Wong C-H., Beutler E., Zimmer K-P., and Kelly J. W.. 2005. Gaucher disease-associated glucocerebrosidases show mutation-dependent chemical chaperoning profiles. Chem. Biol. 12: 1235–1244. [DOI] [PubMed] [Google Scholar]
- 116.Laigre E., Hazelard D., Casas J., Serra-Vinardell J., Michelakakis H., Mavridou I., Aerts J. M., Delgado A., and Compain P.. 2016. Investigation of original multivalent iminosugars as pharmacological chaperones for the treatment of Gaucher disease. Carbohydr. Res. 429: 98–104. [DOI] [PubMed] [Google Scholar]
- 117.Kornhaber G. J., Tropak M. B., Maegawa G. H., Tuske S. J., Coales S. J., Mahuran D. J., and Hamuro Y.. 2008. Isofagomine induced stabilization of glucocerebrosidase. ChemBioChem. 9: 2643–2649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Ben Bdira F., Jiang J., Kallemeijn W., de Haan A., Florea B. I., Bleijlevens B., Boot R., Overkleeft H. S., Aerts J. M., and Ubbink M.. 2016. Hydrophobic interactions contribute to conformational stabilization of endoglycoceramidase II by mechanism-based probes. Biochemistry. 55: 4823–4835. [DOI] [PubMed] [Google Scholar]
- 119.Cox T., Lachmann R., Hollak C., Aerts J., van Weely S., Hrebícek M., Platt F., Butters T., Dwek R., and Moyses C.. 2000. Novel oral treatment of Gaucher’s disease with N-butyldeoxynojirimycin (OGT 918) to decrease substrate biosynthesis. Lancet. 355: 1481–1485. [DOI] [PubMed] [Google Scholar]
- 120.Elstein D., Hollak C., Aerts J., Van Weely S., Maas M., Cox T., Lachmann R., Hrebicek M., Platt F., and Butters T.. 2004. Sustained therapeutic effects of oral miglustat (Zavesca, N-butyldeoxynojirimycin, OGT 918) in type I Gaucher disease. J. Inherit. Metab. Dis. 27: 757–766. [DOI] [PubMed] [Google Scholar]
- 121.Platt F. M., Jeyakumar M., Andersson U., Priestman D., Dwek R., Butters T., Cox T., Lachmann R., Hollak C., and Aerts J.. 2001. Inhibition of substrate synthesis as a strategy for glycolipid lysosomal storage disease therapy. J. Inherit. Metab. Dis. 24: 275–290. [DOI] [PubMed] [Google Scholar]
- 122.Ichikawa S., Sakiyama H., Suzuki G., Hidari K., and Hirabayashi Y.. 1996. Expression cloning of a cDNA for human ceramide glucosyltransferase that catalyzes the first glycosylation step of glycosphingolipid synthesis. Proc. Natl. Acad. Sci. USA. 93: 4638–4643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Abian O., Alfonso P., Velazquez-Campoy A., Giraldo P., Pocovi M., and Sancho J.. 2011. Therapeutic strategies for Gaucher disease: miglustat (NB-DNJ) as a pharmacological chaperone for glucocerebrosidase and the different thermostability of velaglucerase alfa and imiglucerase. Mol. Pharm. 8: 2390–2397. [DOI] [PubMed] [Google Scholar]
- 124.Sawkar A. R., Cheng W-C., Beutler E., Wong C-H., Balch W. E., and Kelly J. W.. 2002. Chemical chaperones increase the cellular activity of N370S β-glucosidase: a therapeutic strategy for Gaucher disease. Proc. Natl. Acad. Sci. USA. 99: 15428–15433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Wennekes T., van den Berg R. J., Boot R. G., van der Marel G. A., Overkleeft H. S., and Aerts J. M.. 2009. Glycosphingolipids—nature, function, and pharmacological modulation. Angew. Chem. Int. Ed. Engl. 48: 8848–8869. [DOI] [PubMed] [Google Scholar]
- 126.Overkleeft H. S., Renkema G. H., Neele J., Vianello P., Hung I. O., Strijland A., van der Burg A. M., Koomen G-J., Pandit U. K., and Aerts J. M.. 1998. Generation of specific deoxynojirimycin-type inhibitors of the non-lysosomal glucosylceramidase. J. Biol. Chem. 273: 26522–26527. [DOI] [PubMed] [Google Scholar]
- 127.Ashe K. M., Bangari D., Li L., Cabrera-Salazar M. A., Bercury S. D., Nietupski J. B., Cooper C. G., Aerts J. M., Lee E. R., and Copeland D. P.. 2011. Iminosugar-based inhibitors of glucosylceramide synthase increase brain glycosphingolipids and survival in a mouse model of Sandhoff disease. PLoS One. 6: e21758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Marques A. R., Aten J., Ottenhoff R., van Roomen C. P., Moro D. H., Claessen N., Veloz M. F. V., Zhou K., Lin Z., and Mirzaian M.. 2015. Reducing GBA2 activity ameliorates neuropathology in Niemann-Pick type C mice. PLoS One. 10: e0135889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Mistry P. K., Liu J., Sun L., Chuang W-L., Yuen T., Yang R., Lu P., Zhang K., Li J., and Keutzer J.. 2014. Glucocerebrosidase 2 gene deletion rescues type 1 Gaucher disease. Proc. Natl. Acad. Sci. USA. 111: 4934–4939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Wennekes T., Meijer A. J., Groen A. K., Boot R. G., Groener J. E., van Eijk M., Ottenhoff R., Bijl N., Ghauharali K., and Song H.. 2010. Dual-action lipophilic iminosugar improves glycemic control in obese rodents by reduction of visceral glycosphingolipids and buffering of carbohydrate assimilation. J. Med. Chem. 53: 689–698. [DOI] [PubMed] [Google Scholar]
- 131.Ghisaidoobe A. T., van den Berg R. J., Butt S. S., Strijland A., Donker-Koopman W. E., Scheij S., van den Nieuwendijk A. M., Koomen G-J., van Loevezijn A., and Leemhuis M.. 2014. Identification and development of biphenyl substituted iminosugars as improved dual glucosylceramide synthase/neutral glucosylceramidase inhibitors. J. Med. Chem. 57: 9096–9104. [DOI] [PubMed] [Google Scholar]
- 132.Aerts J. M., Ottenhoff R., Powlson A. S., Grefhorst A., van Eijk M., Dubbelhuis P. F., Aten J., Kuipers F., Serlie M. J., and Wennekes T.. 2007. Pharmacological inhibition of glucosylceramide synthase enhances insulin sensitivity. Diabetes. 56: 1341–1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Lombardo E., van Roomen C. P., van Puijvelde G. H., Ottenhoff R., van Eijk M., Aten J., Kuiper J., Overkleeft H. S., Groen A. K., and Verhoeven A. J.. 2012. Correction of liver steatosis by a hydrophobic iminosugar modulating glycosphingolipids metabolism. PLoS One. 7: e38520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.van Eijk M., Aten J., Bijl N., Ottenhoff R., van Roomen C. P., Dubbelhuis P. F., Seeman I., Ghauharali-van der Vlugt K., Overkleeft H. S., and Arbeeny C.. 2009. Reducing glycosphingolipid content in adipose tissue of obese mice restores insulin sensitivity, adipogenesis and reduces inflammation. PLoS One. 4: e4723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Langeveld M., and Aerts J. M.. 2009. Glycosphingolipids and insulin resistance. Prog. Lipid Res. 48: 196–205. [DOI] [PubMed] [Google Scholar]
- 136.Aerts J. M., Boot R. G., van Eijk M., Groener J., Bijl N., Lombardo E., Bietrix F. M., Dekker N., Groen A. K., Ottenhoff R., et al. . 2011. Glycosphingolipids and insulin resistance. Adv. Exp. Med. Biol. 721: 99–119. [DOI] [PubMed] [Google Scholar]
- 137.Atsumi S., Umezawa K., Iinuma H., Naganawa H., Nakamura H., Iitaka Y., and Takeuchi T.. 1990. Production, isolation and structure determination of a novel beta-glucosidase inhibitor, cyclophellitol, from Phellinus sp. J. Antibiot. (Tokyo). 43: 49–53. [DOI] [PubMed] [Google Scholar]
- 138.Atsumi S., Nosaka C., Iinuma H., and Umezawa K.. 1992. Inhibition of glucocerebrosidase and induction of neural abnormality by cyclophellitol in mice. Arch. Biochem. Biophys. 297: 362–367. [DOI] [PubMed] [Google Scholar]
- 139.Atsumi S., Nosaka C., Iinuma H., and Umezawa K.. 1993. Accumulation of tissue glucosylsphingosine in Gaucher-like mouse induced by the glucosylceramidase inhibitor cyclophellitol. Arch. Biochem. Biophys. 304: 302–304. [DOI] [PubMed] [Google Scholar]
- 140.Legler G. 1990. Glycoside hydrolases: mechanistic information from studies with reversible and irreversible inhibitors. Adv. Carbohydr. Chem. Biochem. 48: 319–384. [DOI] [PubMed] [Google Scholar]
- 141.Withers S. G., and Aebersold R.. 1995. Approaches to labeling and identification of active site residues in glycosidases. Protein Sci. 4: 361–372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Willems L. I., Jiang J., Li K. Y., Witte M. D., Kallemeijn W. W., Beenakker T. J., Schröder S. P., Aerts J. M., van der Marel G. A., and Codée J. D.. 2014. From covalent glycosidase inhibitors to activity-based glycosidase probes. Chemistry. 20: 10864–10872. [DOI] [PubMed] [Google Scholar]
- 143.Quaroni A., Gershon E., and Semenza G.. 1974. Affinity labeling of the active sites in the sucrase-isomaltase complex from small intestine. J. Biol. Chem. 249: 6424–6433. [PubMed] [Google Scholar]
- 144.Rempel B. P., and Withers S. G.. 2008. Covalent inhibitors of glycosidases and their applications in biochemistry and biology. Glycobiology. 18: 570–586. [DOI] [PubMed] [Google Scholar]
- 145.Hermans M. M., Kroos M., Van Beeumen J., Oostra B., and Reuser A.. 1991. Human lysosomal alpha-glucosidase. Characterization of the catalytic site. J. Biol. Chem. 266: 13507–13512. [PubMed] [Google Scholar]
- 146.Herrchen M., and Legler G.. 1984. Identification of an essential carboxylate group at the active site of lacZβ-galactosidase from Escherichia coli. Eur. J. Biochem. 138: 527–531. [DOI] [PubMed] [Google Scholar]
- 147.Gebler J. C., Aebersold R., and Withers S.. 1992. Glu-537, not Glu-461, is the nucleophile in the active site of (lac Z) beta-galactosidase from Escherichia coli. J. Biol. Chem. 267: 11126–11130. [PubMed] [Google Scholar]
- 148.Miao S., McCarter J. D., Grace M. E., Grabowski G. A., Aebersold R., and Withers S. G.. 1994. Identification of Glu340 as the active-site nucleophile in human glucocerebrosidase by use of electrospray tandem mass spectrometry. J. Biol. Chem. 269: 10975–10978. [PubMed] [Google Scholar]
- 149.Caron G., and Withers S.. 1989. Conduritol aziridine: a new mechanism-based glucosidase inactivator. Biochem. Biophys. Res. Commun. 163: 495–499. [DOI] [PubMed] [Google Scholar]
- 150.Sulzenbacher G., Schülein M., and Davies G. J.. 1997. Structure of the endoglucanase I from Fusarium oxysporum: native, cellobiose, and 3, 4-epoxybutyl β-D-cellobioside-inhibited forms, at 2.3 Å resolution. Biochemistry. 36: 5902–5911. [DOI] [PubMed] [Google Scholar]
- 151.Havukainen R., Törrönen A., Laitinen T., and Rouvinen J.. 1996. Covalent binding of three epoxyalkyl xylosides to the active site of endo-1, 4-xylanase II from Trichoderma reesei. Biochemistry. 35: 9617–9624. [DOI] [PubMed] [Google Scholar]
- 152.Stephens M. C., Bernatsky A., Burachinsky V., Legler G., and Kanfer J.. 1978. The Gaucher mouse: differential action of conduritol B epoxide and reversibility of its effects. J. Neurochem. 30: 1023–1027. [DOI] [PubMed] [Google Scholar]
- 153.Vardi A., Zigdon H., Meshcheriakova A., Klein A. D., Yaacobi C., Eilam R., Kenwood B. M., Rahim A. A., Massaro G., and Merrill A. H.. 2016. Delineating pathological pathways in a chemically induced mouse model of Gaucher disease. J. Pathol. 239: 496–509. [DOI] [PubMed] [Google Scholar]
- 154.Hays W. S., Wheeler D. E., Eghtesad B., Glew R. H., and Johnston D. E.. 1998. Expression of cytosolic β-glucosidase in guinea pig liver cells. Hepatology. 28: 156–163. [DOI] [PubMed] [Google Scholar]
- 155.Morjani H., Aouali N., Belhoussine R., Veldman R. J., Levade T., and Manfait M.. 2001. Elevation of glucosylceramide in multidrug-resistant cancer cells and accumulation in cytoplasmic droplets. Int. J. Cancer. 94: 157–165. [DOI] [PubMed] [Google Scholar]
- 156.Gloster T. M., Madsen R., and Davies G. J.. 2007. Structural basis for cyclophellitol inhibition of a β-glucosidase. Org. Biomol. Chem. 5: 444–446. [DOI] [PubMed] [Google Scholar]
- 157.Witte M. D., Kallemeijn W. W., Aten J., Li K-Y., Strijland A., Donker-Koopman W. E., Van Den Nieuwendijk A. M., Bleijlevens B., Kramer G., Florea B. I., et al. . 2010. Ultrasensitive in situ visualization of active glucocerebrosidase molecules. Nat. Chem. Biol. 6: 907–913. [DOI] [PubMed] [Google Scholar]
- 158.Kallemeijn W. W., Li K. Y., Witte M. D., Marques A. R., Aten J., Scheij S., Jiang J., Willems L. I., Voorn-Brouwer T. M., and van Roomen C. P.. 2012. Novel activity-based probes for broad-spectrum profiling of retaining β-exoglucosidases in situ and in vivo. Angew. Chem. Int. Ed. Engl. 51: 12529–12533. [DOI] [PubMed] [Google Scholar]
- 159.Kallemeijn W. W., Witte M. D., Wennekes T., and Aerts J.. 2014. Mechanism-based inhibitors of glycosidases: design and applications. Adv. Carbohydr. Chem. Biochem. 71: 297–338. [DOI] [PubMed] [Google Scholar]
- 160.Willems L. I., Beenakker T. J., Murray B., Gagestein B., van den Elst H., van Rijssel E. R., Codée J. D., Kallemeijn W. W., Aerts J. M., and van der Marel G. A.. 2014. Synthesis of α-and β-galactopyranose-configured isomers of cyclophellitol and cyclophellitol aziridine. Eur. J. Org. Chem. 2014: 6044–6056. [Google Scholar]
- 161.Jiang J., Kuo C-L., Wu L., Franke C., Kallemeijn W. W., Florea B. I., van Meel E., van der Marel G. A., Codée J. D., and Boot R. G.. 2016. Detection of active mammalian GH31 α-glucosidases in health and disease using in-class, broad-spectrum activity-based probes. ACS Cent. Sci. 2: 351–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Jiang J., Kallemeijn W. W., Wright D. W., van den Nieuwendijk A. M., Rohde V. C., Folch E. C., van den Elst H., Florea B. I., Scheij S., and Donker-Koopman W. E.. 2015. In vitro and in vivo comparative and competitive activity-based protein profiling of GH29 α-L-fucosidases. Chem. Sci. 6: 2782–2789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Marques A. R., Willems L. I., Herrera Moro D., Florea B. I., Scheij S., Ottenhoff R., van Roomen C. P., Verhoek M., Nelson J. K., and Kallemeijn W. W.. 2017. A specific activity-based probe to monitor family GH59 galactosylceramidase, the enzyme deficient in Krabbe disease. ChemBioChem. 18: 402–412. [DOI] [PubMed] [Google Scholar]
- 164.Wu L., Jiang J., Jin Y., Kallemeijn W. W., Kuo C-L., Artola M., Dai W., van Elk C., van Eijk M., and van der Marel G. A.. 2017. Activity-based probes for functional interrogation of retaining β-glucuronidases. Nat. Chem. Biol. 13: 867–873. [DOI] [PubMed] [Google Scholar]
- 165.Herrera Moro Chao D., Kallemeijn W. W., Marques A. R., Orre M., Ottenhoff R., van Roomen C., Foppen E., Renner M. C., Moeton M., and van Eijk M.. 2015. Visualization of active glucocerebrosidase in rodent brain with high spatial resolution following in situ labeling with fluorescent activity based probes. PLoS One. 10: e0138107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Lahav D., Liu B., van den Berg R. J., van den Nieuwendijk A. M., Wennekes T., Ghisaidoobe A. T., Breen I., Ferraz M. J., Kuo C-L., and Wu L.. 2017. A fluorescence polarization activity-based protein profiling assay in the discovery of potent, selective inhibitors for human non-lysosomal glucosylceramidase. J. Am. Chem. Soc. 139: 14192–14197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Artola M., Wu L., Ferraz M. J., Kuo C-L., Raich L., Breen I. Z., Offen W. A., Codée J. D. C., van der Marel G. A., Rovira C., et al. . 2017. 1,6-Cyclophellitol cyclosulfates: a new class of irreversible glycosidase inhibitor. ACS Cent. Sci. 3: 784–793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Wicki J., Rose D. R., and Withers S. G.. 2002. Trapping covalent intermediates on beta-glycosidases. Methods Enzymol. 354: 84–105. [DOI] [PubMed] [Google Scholar]
- 169.Blanchard J. E., and Withers S. G.. 2001. Rapid screening of the aglycone specificity of glycosidases: applications to enzymatic synthesis of oligosaccharides. Chem. Biol. 8: 627–633. [DOI] [PubMed] [Google Scholar]
- 170.Vocadlo D. J., Davies G. J., Laine R., and Withers S. G.. 2001. Catalysis by hen egg-white lysozyme proceeds via a covalent intermediate. Nature. 412: 835–838. [DOI] [PubMed] [Google Scholar]
- 171.Withers S. G., and Street I. P.. 1988. Identification of a covalent. alpha-D-glucopyranosyl enzyme intermediate formed on a. beta.-glucosidase. J. Am. Chem. Soc. 110: 8551–8553. [Google Scholar]
- 172.McMahon L. G., Nakano H., Levy M-D., and Gregory J. F.. 1997. Cytosolic pyridoxine-β-d-glucoside hydrolase from porcine jejunal mucosa. Purification, properties, and comparison with broad specificity β-glucosidase. J. Biol. Chem. 272: 32025–32033. [DOI] [PubMed] [Google Scholar]
- 173.Arribas J. C. D., Herrero A. G., Martín-Lomas M., Cañada F. J., He S., and Withers S. G.. 2000. Differential mechanism-based labeling and unequivocal activity assignment of the two active sites of intestinal lactase/phlorizin hydrolase. Eur. J. Biochem. 267: 6996–7005. [DOI] [PubMed] [Google Scholar]
- 174.Vocadlo D. J., and Bertozzi C. R.. 2004. A strategy for functional proteomic analysis of glycosidase activity from cell lysates. Angew. Chem. Int. Ed. Engl. 43: 5338–5342. [DOI] [PubMed] [Google Scholar]
- 175.Hekmat O., Kim Y-W., Williams S. J., He S., and Withers S. G.. 2005. Active-site peptide “fingerprinting” of glycosidases in complex mixtures by mass spectrometry discovery of a novel retaining β-1, 4-glycanase in Cellulomonas fimi. J. Biol. Chem. 280: 35126–35135. [DOI] [PubMed] [Google Scholar]