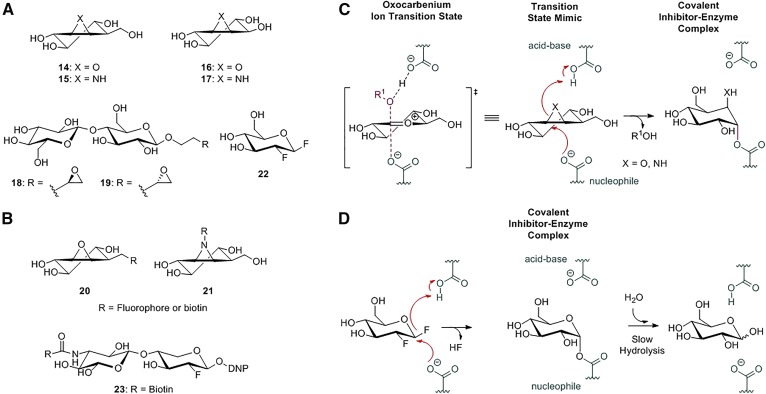
Fig. 6.
Retaining β-glycosidase mechanism-based covalent inhibitors and ABPs. A: Chemical structures of epoxide-, aziridine-, and fluorinated-based inactivators: cyclophellitol (14), cyclophellitol aziridine (15), conduritol B (16), conduritol aziridine (17), exo-alkyl epoxide glycosides (18 and 19), and 2-deoxy-2-fluoro glucoside (22). B: ABPs targeting glycosidases: cyclophellitol (aziridine) ABPs (20 and 21) and 2-deoxy-2-fluoro glycoside-based ABP (23). C: Cyclophellitol inactivation mechanism: cyclophellitol analogues irreversibly inhibit glycosidases by mimicking the oxocarbenium ion transition state and employing epoxides or aziridines as an electrophilic trap. D: 2-Deoxy-2-fluoro glucoside inactivators irreversibly inhibit glycosidases, forming a covalent inhibitor-enzyme complex that is subsequently slowly hydrolyzed.