Abstract
Context
Modifiable factors that reduce the burden of the metabolic syndrome (MetS), particularly plant-derived biomarkers, have been a recent focus of rising interest.
Objective
This systematic review and meta-analysis, which follows PRISMA guidelines, evaluates evidence from a period of 20 years that links vitamin A and carotenoids with the occurrence of MetS and following the PRISMA guidelines.
Data Sources
PubMed and Cochrane databases (January 1997 through March 2017) were systematically assessed for studies, including case–control, cross-sectional, and cohort studies, that evaluated the associations of MetS with carotenoids and retinyl esters and retinol (vitamin A).
Data Extraction
Key measures of associations were harmonized into odds ratios (ORs) and 95% confidence intervals (95%CI) of MetS per 1 standard deviation (SD) of exposure using forest plots and random effects models that pooled data points from 11 cross-sectional studies. Begg’s funnel and harvest plots were constructed.
Results
An inverse association between total carotenoids and MetS was found [ORpooled, 0.66; 95%CI, 0.56–0.78; 1 SD ∼ 0.82 µmol/L; n = 5 studies]. This association was the strongest for β-carotene, followed by α-carotene and β-crypotoxanthin. No association was detected between retinol and MetS (ORpooled, 1.00; 95%CI, 0.88–1.13; 1 SD ∼ 2.14 µmol/L; n = 6 studies). Publication bias was absent, and harvest plots indicated consistency upon replication for β-carotene and total carotenoid exposures.
Conclusions
This review and meta-analysis suggests that, unlike retinol, total and individual carotenoids were inversely related to MetS.
Keywords: carotenoids, meta-analysis, metabolic syndrome, vitamin A
INTRODUCTION
The metabolic syndrome (MetS) is often defined by the clustering of cardiometabolic abnormalities that include anthropometric and physiological parameters with preset criteria recognized by bodies such as the World Health Organization (WHO), the National Cholesterol Education Program–Adult Treatment Panel III (NCEP–ATP III), and the International Diabetes Federation (IDF).1–5
The coexistence of excess abdominal adiposity with hyperglycemia, elevated blood pressure, lower concentration of high-density lipoprotein cholesterol, and hypertriglyceridemia ultimately increases the risk of type 2 diabetes (T2D) and cardiovascular disease (CVD) by 5-fold and 1.7-fold, respectively.1,2,6,7 Thus, MetS is a major threat to public health, increasing all-cause mortality rates, disability, and healthcare costs,8–16 with evidence suggesting that its prevalence has been on the rise over the past several decades.17,18 Over the years, interest has grown in modifiable lifestyle factors that would reduce the burden of MetS, particularly dietary factors and their intermediary nutritional biomarkers.19–22 Of particular interest is the role of carotenoids and their conversion products (retinoids) in adipogenesis, lipolysis, insulin resistance, and the pathophysiology of MetS.23
In addition, potential beneficial anti-inflammatory and antioxidant effects have recently been ascribed to naturally occurring plant-derived micronutrients. These micronutrients may reduce oxidative stress triggered by injury, which characterizes the pathogenesis of numerous chronic diseases, including MetS, T2D, CVD, rheumatological conditions, and carcinogenesis.24 Serum concentrations of micronutrients reflect their intake in the diet according to recent studies.25,26 Among those antioxidants, carotenoids have been the focus of recent interest. Although mostly found in fruits and vegetables, carotenoids are available in other sources, such as bread, eggs, beverages, fats, and oils.27 Out of > 40 carotenoids in the human diet, only β-carotene, α-carotene, β-cryptoxanthin, lycopene, lutein, and zeaxanthin are ubiquitous in human serum.27 Some observational studies showed inverse associations between carotenoids and CVD28 and T2D,29–33 but findings were inconsistent.28,34–37 The main known function of carotenoids in humans is to serve as precursors for retinol (vitamin A), retinaldehyde, and retinoic acid, among other retinoid conversion products that play important roles as transcriptional regulators in the visual cycle and gene regulation linked to many developmental and physiological processes.38,39
The roles of serum carotenoid and its retinoid conversion products in the etiology of MetS have not been systematically reviewed up to this point, particularly in a quantitative meta-analysis. This systematic review and meta-analysis attempts to pool, interpret, and evaluate research evidence from the the past 20 years linking serum carotenoids, retinyl esters, and retinol with the occurrence of MetS. This study also reviews qualitative evidence of an association between MetS and dietary carotenoids (Table 1).
Table 1.
PICOS criteria for inclusion and exclusion of studies
| Parameters | Inclusion criteria | Exclusion criteria |
|---|---|---|
| Population | General population of adults and adolescents aged > 12 y | Aged <12 y |
| Intervention, prognostic factor, or exposure | Total or individual levels of carotenoids (diet or serum), serum retinol, or retinyl esters available | Dietary intakes do not include carotenoids and/or serum level exposures do not include carotenoids, retinol or retinyl esters |
| Comparison | Continuous exposure; z scores, quantiles that can be converted to z scores | Binary exposures |
| Outcome | Metabolic syndrome outcome | Individual components of the metabolic syndrome were not studied |
| Study design | All types of observational studies were included in the systematic review; meta-analysis was done on the most common design (cross-sectional studies) | Case–control and prospective cohort studies were excluded from the meta-analysis. |
METHODS
Search strategy
Using PubMed and Endnote version X8, a systematic review of the literature on the association of MetS with carotenoids and vitamin A was conducted. This search was supplemented with a comparable search using the Cochrane database. Original research that was published between January 1, 1997 and March 31, 2017 was considered; the starting date of January 1, 1997 was chosen because MetS was rarely studied prior to 1997. After an initial search of abstracts using combinations of “MetS” with pertient keywords (ie, carotenoid, carotene, lutein, zeaxanthin, cryptoxanthin, lycopene, vitamin A, retinol, retinyl), retrieved papers were assessed for relevance by review of the titles and abstracts. Key information, such as study design, contextual setting, sample size, main outcome, and key findings, was retrieved from the articles selected for review. The PRISMA guidelines were followed, and the related checklist has been provided (Appendix S1 in the Supporting Information online).
Study identification and selection
Two independent reviewers determined whether studies were selected for review and meta-analysis. Final inclusion criteria were the following: 1) Study design was case–control, cross-sectional, or cohort study (thus review articles, commentaries, and basic science papers were excluded); 2) The main outcome was MetS, primarily defined using the NCEP–ATP III criteria, though other criteria may be used. The MetS components were described in the findings of individual studies but were not subjected to further meta-analysis. Thus, only studies that examined MetS in relation to the key exposures were included. 3) Only studies with the most common study design (ie, cross-sectional) were included in the meta-analysis. Non-English language citations were excluded. Case–control, cross-sectional, and cohort studies that were selected specifically for review and potential meta-analysis are presented in Table 2.1,18,40–52
Table 2.
Summary of studies selected for the meta-analysis of serum carotenoids, vitamin A, and the metabolic syndrome, PubMed and Cochrane database search 1997–2017
| References | Country | Study design | Serum exposure measure(s) | Total sample size | Female, % | Age at baseline | Population | Quality score |
|---|---|---|---|---|---|---|---|---|
| Carotenoids only | ||||||||
| Sugiura et al (2008)40 | Japan | Cross-sectional | Carotene, retinol, tocopherol, lycopene, lutein, cryptoxanthin, zeaxanthin | 1073 | 66.7 | 30–70 y | Japanese from town of Mikkabi, Japan | 6 |
| Coyne et al (2009)41 | Australia | Cross-sectional | Total carotenoids and individual carotenoids (α- carotene, β-carotene, β-cryptoxanthin, lutein, zeaxanthin, lycopene) | 1523 | 57.9 | ≥25 y | Australian from Queensland, Australia | 6 |
| Czernichow et al (2009)42 | France | Randomized controlled trial | Serum antioxidant supplements (β-carotene, vitamin C, vitamin E, zinc, selenium) | 5220 |
|
|
French population | 8 |
| Suzuki et al (2011)43 | Japan | Cross-sectional | β-cryptoxanthin, carotene, zeaxanthin, lutein | 931 | 65.8 | 39–70 y | Japanese from town of Yakumo, Japan | 6 |
| Liu et al (2014)44 | China | Cross-sectional | Total and individual carotenoids, α-tocopherol, retinol | 2148 | 72.0 | 50–75 y | Middle-aged and elderly Chinese from Urban Guangzhou, China | 4 |
| Sugiura et al (2015)45 | Japan | Longitudinal cohort | Carotenoids (lutein, lycopene, α- carotene, β-carotene, cryptoxanthin, zeaxanthin) | 910 | 67.6 |
|
Japanese from town of Mikkabi, Japan | 7 |
| 6 | ||||||||
| Han et al (2016)46 | USA | Cross-sectional | Serum lycopene | 13 196 | 52.0 | ≥20 y | NHANES 2001–2006 | |
| Vitamin A only | ||||||||
| Sharma et al (2005)47 | India | Cross-sectional | Vitamin A, vitamin C, and vitamin E | 544 | NA |
|
Urban Indian families | 2 |
| Suriyaprom et al (2014)48 | Thailand | Cross-sectional | Homocysteine, amyloid A, vitamin A, and vitamin E | 405 | 100 | 58.0 (SD: 5.9) y | Postmenopausal Thai women | 2 |
| Godala et al (2016)49 | Poland | Case–control | Vitamin A, vitamin C, and vitamin E |
|
|
|
Study population from Lodz, Poland | 2 |
| Both | ||||||||
| Molnar et al (2004)50 | Hungary | Case–control | Retinol, tocopherol, β-carotene | 48 | 33.3 |
|
Hungarian children from Pecs, Hungary | 2 |
| Ford et al (2003)1 | USA | Cross-sectional | Total and individual carotenoids, retinol, retinyl esters | 8808 |
|
≥20 y [MetS: 54.3 (SE: 0.7) y; Non-MetS: 41.0 (SE: 0.4) y] | NHANES 1988–1994, a representative sample of noninstitutionalized civilian US population, majority was white (MetS: 79.7%, non-MetS: 75.5%) | 7 |
| Beydoun et al (2011)18 | USA | Cross-sectional | Total and individual carotenoids, retinol, retinyl esters | 11 845 | 50.7 | 20–85 y | NHANES 2001–2006, a representative sample of noninstitutionalized civilian US population, majority was white | 7 |
| Beydoun et al (2012)51 | USA | Cross-sectional | Total and individual carotenoids, retinol, retinyl esters | 1339 | 50.0 | 12–19 y | NHANES 2001–2006, a representative sample of noninstitutionalized civilian US population, majority was white | 6 |
| Li et al (2013)52 | China | Cross-sectional | Serum superoxide dismutase, glutathione peroxidase, malondialdehide, vitamin A, vitamin E, β-carotene, lycopene | 550 | 61.6 | 18–65 y | Chinese from Shanghai, China | 4 |
The metabolic syndrome was defined based on standard criteria, using measured parameters in each study that was included. Shaded studies are studies not included in the quantitative meta-analysis, due to a study design not comparable with cross-sectional studies (eg, cohort, case–control, and randomized controlled trial).
Abbreviations: NA, not available; SD, standard deviation; SE, standard error; MetS, metabolic syndrome; NHANES, National Health and Nutrition Examination Surveys.
Data extraction
Table 2 presents a summary of the studies, including study location, participant characteristics (ie, age, sex), study design (ie, case–control, cross-sectional, cohort), sample size (ie, number of cases and controls, or total sample size), type of serum exposures measured, type of population, and quality score (Appendix S2 in the Supporting Information online).
Meta-analysis
Focusing on data points with incident or prevalent MetS as an outcome, a meta-analysis was conducted to assess the strength of the association of MetS outcomes with selected serum carotenoid (total carotenoids, and individual carotenoids when available) and retinol (vitamin A) and retinyl esters exposures. This analysis was restricted to cross-sectional studies with available data that had comparable measurements for each risk/protective factor, thus allowing the estimation of a pooled measure of association across those data and studies. To have a larger number of studies that could be included, standardized mean differences (Cohen’s D) were also used and converted to Loge (odds ratios [OR]), which were then reconverted to ORs with associated 95% confidence intervals (95%CIs).53 Although this transformation was mostly applied to case–control studies, a few of the included cross-sectional studies underwent this transformation. In those studies, means of serum carotenoids or retinol and retinyl esters exposures were compared between MetS (MetS+) and no MetS (MetS−) groups, in some cases using multiple linear regression to test differences while adjusting for key confounders. Other modifications to the ORs were made when measurements were done on different scales of exposure (eg, per 1 standard deviation [SD] vs quartiles vs tertile vs per 1 unit [eg, 1 µmol/L] increase). All ORs were converted into a single measure of association that closely represents the effect of 1 SD increase in the exposure on the risk of MetS. After converting the ORs with their 95%CIs to LogeOR with its standard error (SE), both parameters (ie, the point estimate and its SE) were divided by a conversion factor. In fact, in a normal distribution, the means of the highest and lowest tertile lie 2.18 SDs apart; therefore, the log ORs were divided by 2.18 to obtain log OR per SD. Similarly, extreme quintiles effects were divided by 2.8, and extreme quartile effects were divided by 2.54. This approach has been adopted elsewhere.21 Finally, the value of the SD (in µmol/L of carotenoids or retinol [vitamin A]) was estimated as the average of SDs of studies that were included in each analysis and was reported in each forest plot.53
The study-specific ORs for each exposure of interest were then pooled using random effects models, after testing for heterogeneity using the I2 test.53 As such, a summary or pooled OR was provided using forest plots and estimated using inverse variance weighting.54 Random effects models that further incorporated between-study variability were conducted using DerSimonian and Laird’s methodology.54 In some cases, where measures of association were estimated for 2 correlated exposures (eg, lutein only and zeaxanthin only), a preliminary pooling was made to estimate the OR for lutein+zeaxanthin versus MetS.
Harvest and funnel plots
A predefined quality score (QS) was used to assess the quality of each included study. This QS is a modified version of previously used scoring systems55 and was applied in a previous meta-analysis.21 In this meta-analysis, the QS scale included 4 items—namely, study design, study size, outcome assessment, and adjustment for potential confounders, each of which can be scored from 0 to 2 in ascending order of quality. Thus, the total QS score could range from 0 to 8. Since only 1 outcome was studied (ie, MetS), only 1 QS was linked to each study. Three independent assessments were made by 3 co-authors, and the average QS was determined. A consensus was then achieved by the 3 co-authors after initial rating. To represent graphically the key findings of studies for each exposure of interest, a harvest plot was used. This plot shows the exposure-outcome associations of interest in each study, whether they were statistically significant, and in which direction (−1 = “inverse association”, 0 = “null association”, 1 = “positive association”) for each exposure against QS, which is presented on the y-axis. At least 3 studies were needed to create a harvest plot per hypothesized exposure–outcome association.56,57
Finally, in order to examine publication bias, Begg’s funnel plots were used; each OR point estimate was plotted against the corresponding SE for each study on a logarithmic scale,58 combining all exposures (eg, carotenoid and vitamin A exposures). This type of bias was also formally tested using the Begg-adjusted rank correlation tests59 and the Egger’s regression asymmetry test.60 All analyses were conducted with STATA 14.0 (StataCorp, College Station, TX, USA), using a suite of meta-analysis commands.61 Type I error was set at 0.05 for all measures of association.
RESULTS
Study selection
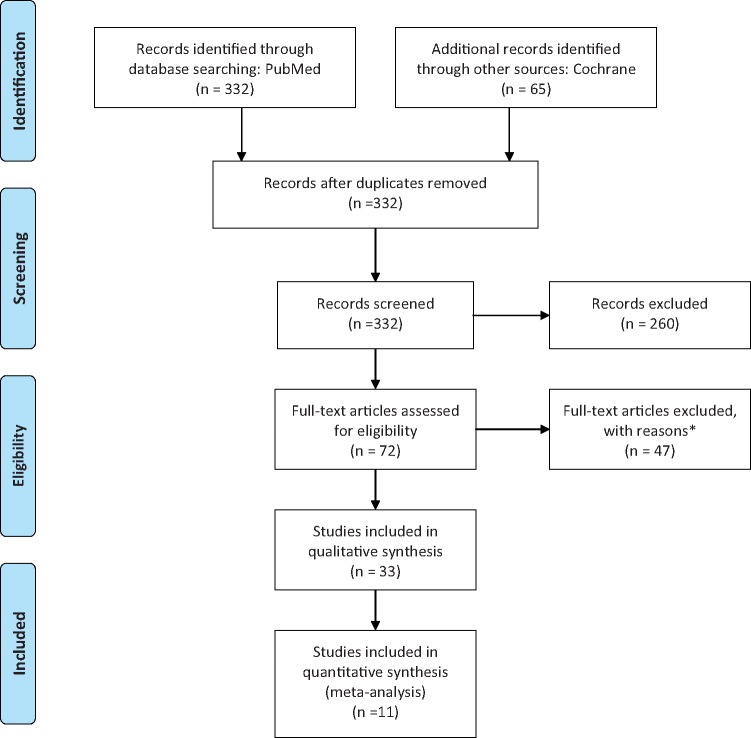
Figure 1 provides a flow diagram of the literature search process, showing the main reasons for exclusion and the final number of studies included for each type of meta-analysis or literature review. Of 332 unduplicated titles and abstracts published between 1997 and 2017 and found through the PubMed search, 41 published original epidemiological studies were considered for review and/or meta-analysis. Of these, 12 were used for the meta-analysis of serum carotenoids versus MetS1,18,40–46,50–52 and 8 were used for the meta-analysis of serum vitamin A (or retinol/retinyl esters) versus MetS1,18,47–52; 5 studies examined both vitamin A and carotenoid exposures. Thus, 15 case–control, cross-sectional, and prospective cohort studies were potentially included in the meta-analysis. Of these, only 11 cross-sectional studies (ie, the most common study design) were included in the final meta-analysis. Of the 41 unduplicated citations that were selected for review or meta-analysis, those investigating the association between dietary carotenoids/vitamin A or related dietary patterns (eg, fruit juices) and MetS (n = 18) were reviewed and summarized without further meta-analysis.45–49,61–73 Moreover, because meta-analysis was reserved for the cross-sectional studies, the case–control, cohort, and randomized controlled trial studies that examined serum concentrations of retinol and carotenoids in relation to MetS (n = 4) were only described qualitatively. The Cochrane database search did not contribute any additional studies.
Figure 1.
Flow diagram of the literature search process. *Main reasons for exclusion were as follows: non-English language (n = 15), animal or basic study (n = 4), reviews (n = 10).
Studies of the associations of serum retinol (vitamin A) and carotenoids with the metabolic syndrome: a qualitative review
Among the 15 studies that were selected for this review, 2 were case–control studies,49,50 11 had a cross-sectional design,1,18,40,41,43,44,46–48,51,52 and 2 were longitudinal studies or randomized controlled trial studies.42,45 Among case–control studies, Godala et al examined the association among serum vitamins A, C, and E and MetS after selecting 182 cases matched with 91 controls, with a mean age of approximately 58 years. All 3 vitamin concentrations were found to be lower among MetS cases than controls.49 Another smaller case–control study found that both absolute and serum β-carotene levels corrected for plasma lipids (cholesterol + triglyceride) were significantly (P < 0.05) lower in obese children with MetS compared with controls who were non-MetS and nonobese, with a similar trend observed for vitamin E and total antioxidant status.50 Among cross-sectional studies, 4 recent studies were conducted using the National Health and Nutrition Survey data from 1988–1994 to 2001–2006.1,18,46,51 Three of the 4 studies concluded that adults with the MetS have suboptimal concentrations of several antioxidants, including serum total carotenoids and β-carotene. The most recent study, which examined effect modification by adiposity measured with weight and height, suggested that body mass index may act as an effect modifier in the association between lycopene and MetS.46 Results from studies suggest that visceral adiposity, a cardinal component of MetS, may contribute to excess RBP4 and serum retinol in obese children, which may explain the direct correlation between serum retinol and MetS in this population and confirms the inverse association for lipid-corrected β-carotene.62 Results from other studies suggest that, among adolescents, serum retinol was positively associated with MetS, though no association was detected in the case of most carotenoids.51 Furthermore, 2 of 3 studies that examined serum retinyl esters as a key exposure found an inverse association with MetS among adults,1,18 whereas the third study, which was conducted among adolescents, did not detect a relationship.51 Overall, 5 cross-sectional studies examined total carotenoids in relation to MetS,1,18,41,44,51 4 of which found an inverse relationship.1,18,41,44 In the most recent such study,44 which was conducted among 2148 adults in China aged 50–75 years, a dose–response inverse relationships between individual serum carotenoid concentrations and total carotenoids and the prevalence of the MetS was found after adjusting for potential confounders (P for trend < 0.001). Specifically, the ORs of the MetS for the highest (vs lowest) quartile were 0.31 (95%CI, 0.20–0.47) for alpha-carotene, 0.23 (95%CI, 0.15–0.36) for β-carotene, 0.44 (95%CI, 0.29–0.67) for β-cryptoxanthin, 0.39 (95%CI, 0.26–0.58) for lycopene, 0.28 (95%CI, 0.18–0.44) for lutein+zeaxanthin, and 0.19 (95%CI, 0.12–0.30) for total carotenoids. In contrast with carotenoids, serum retinol (vitamin A) was not associated with MetS in most selected cross-sectional studies, particularly those conducted among adults.1,18,47,48,52
One longitudinal study of 910 middle-aged and older adults in Japan45 examined the association between baseline serum carotenoids and incident MetS. After adjustments for confounders, the hazard ratio (HR) for the MetS in the highest tertile of serum β-carotene compared with the lowest tertile was 0.47 (95%CI, 0.23–0.95), a relationship mostly driven by the dyslipidemia components of MetS. Within a randomized controlled trial conducted among French adults (SU.VI.MAX),42 it was found that antioxidant supplementation for 7.5 years did not affect the risk of MetS. Nevertheless, baseline serum antioxidant concentrations of β-carotene and vitamin C were inversely associated with the risk of MetS with adjusted ORs for the highest compared with the lowest tertile of 0.34 (95%CI, 0.21–0.53; P for trend = 0.0002) and 0.53 (95%CI, 0.35–0.80; P for trend = 0.01), respectively.
Studies of dietary carotenoids’ association with the metabolic syndrome: a qualitative review
Many studies have corroborated these findings using dietary exposures rather than serum levels of carotenoids, although others have not.42,49,63–70 For instance, a case–control study of 49 MetS cases and 94 non-MetS controls suggested that intake of β-carotene was significantly higher among non-MetS controls (mean ± SD, 3832 ± 1731 µg/d in non-MetS vs 2967 ± 1953 µg/d among MetS cases; P = 0.026), with no differences detected in terms of vitamin A intakes.63 One of the key studies examining this research question surveyed 374 men aged 40–80 years in the Netherlands and found that MetS had a 22% prevalence overall. More important, there was an inverse association between higher total carotenoid intake and the presence of MetS, with a multivariate-adjusted relative risk of highest versus lowest quartile of 0.42 (95%CI, 0.20–0.87). Although β-cryptoxanthin, lutein, and zeaxanthin were not associated with MetS, a clear dose–response association was retained in the multivariate model with lycopene quartile as the predictor of MetS. This dose–response was only observed for α- and β- carotene quartiles in age-adjusted models for MetS.70 Nevertheless, another study found no significant association between carotenoid intake and MetS in a large sample of adults in China (n = 2069; n = 1109 males; 17% MetS prevalence).65 In addition to an inverse relationship between serum β-carotene and MetS among adults in France who participated in a randomized placebo-controlled trial (SU.VI.MAX), antioxidant supplementation was inversely related to MetS incidence after a follow-up time of 7.5 years.42
Meta-analysis
Studies selected for the meta-analysis (n = 11) were published between 2003 and 2016 (mean±SD, 2010 ± 4), with 4 of the studies conducted in the United States, 2 in Japan, 2 in China, 1 in India, 1 in Thailand, and 1 in Australia (Table 2). Ten studies included adults of varying age ranges, whereas 1 included adolescents. Moreover, 6 studies had a balanced male/female composition, whereas the remaining 5 were all/mostly female. The cumulative sample of studies included in this meta-analysis consisted of 29 673 participants, with a mean ± SD of 2697 ± 4283 participants per study. Finally, mean QS with its SD was 5.5 ± 1.9 (range, 2–7), indicating a relatively good quality set of studies, given that the maximum score is 8.
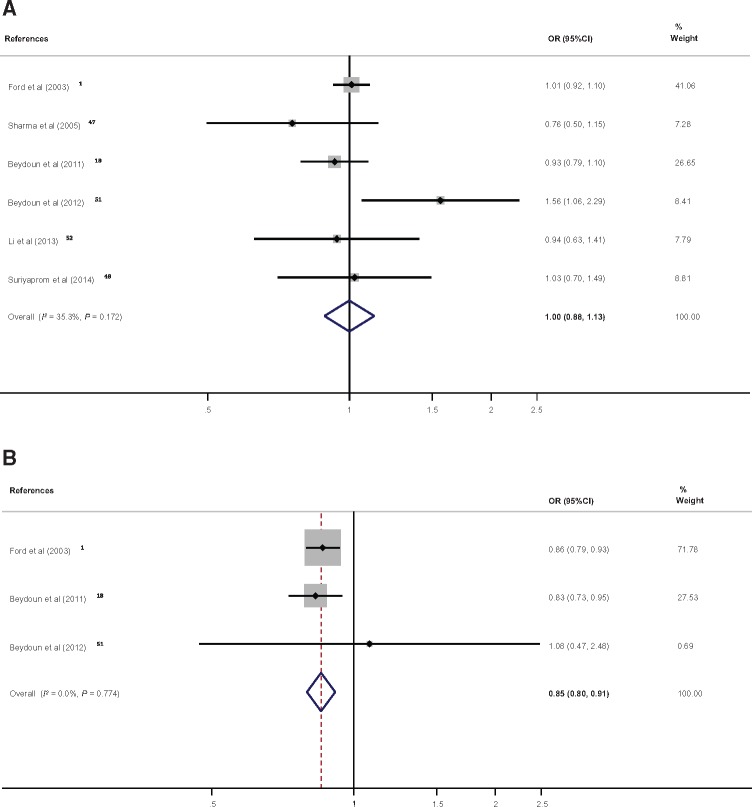
In total, measures of the association of serum retinol and/or retinyl esters with MetS from 6 studies were pooled. The pooled findings indicated that there was no detectable association between serum retinol concentration and MetS occurrence (OR, 1.00; 95%CI, 0.88–1.13; 1 SD ∼ 2.47 µmol/L; n = 6 studies; I2 = 35.3%), whereas, based on data points from 3 studies, serum retinyl esters were shown to have an inverse relationship with the odds of MetS (OR, 0.85; 95%CI, 0.85–91; 1 SD ∼ 0.19 µmol/L; I2 = 0.0%) (Figure 2A and B). The corresponding results in terms of Cohen’s D and its 95%CI are presented in Figures S1A and B in the Supporting Information online.
Figure 2.
Forest plot of odds ratios with 95% confidence intervals of the association between serum vitamin A [per standard deviation of (A) retinol1,18,47,48,51,52 or (B) retinyl ester1,18,51] and the metabolic syndrome, 1997–2017. A, One standard deviation of retinol was estimated on average at 2.14 µmol/L with a standard error of 1.32; B, One standard deviation of retinyl esters was estimated on average at 0.19 µmol/L with a standard error of 0.12.
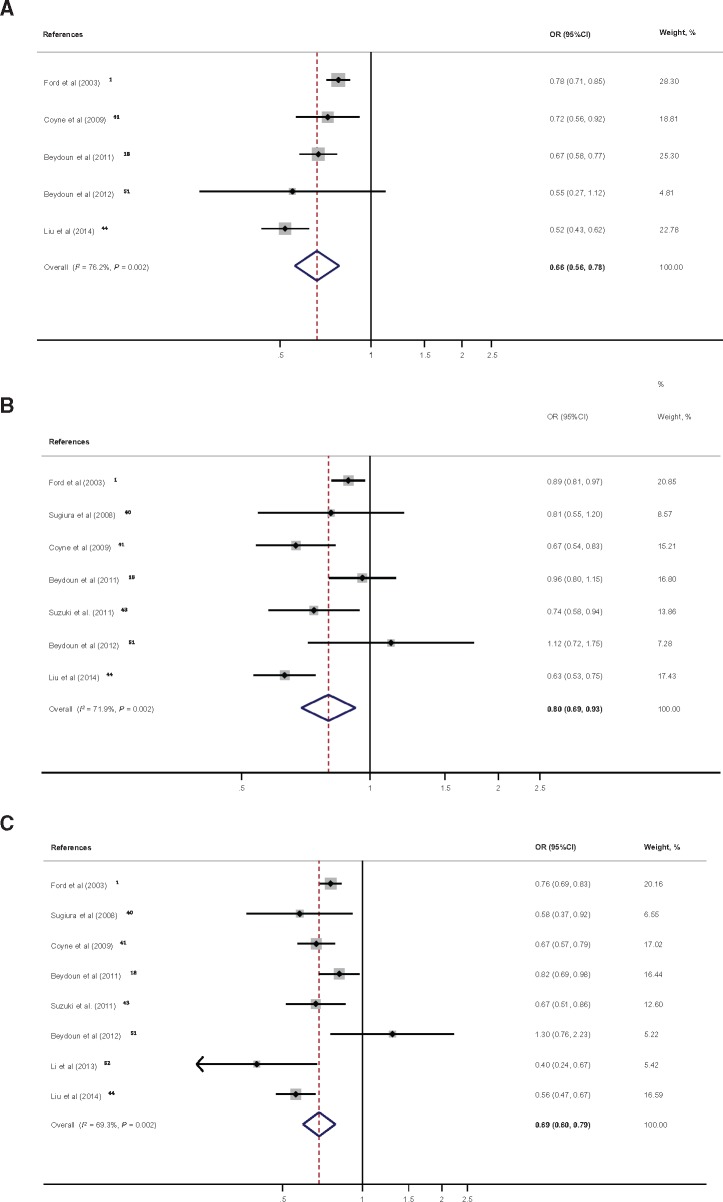
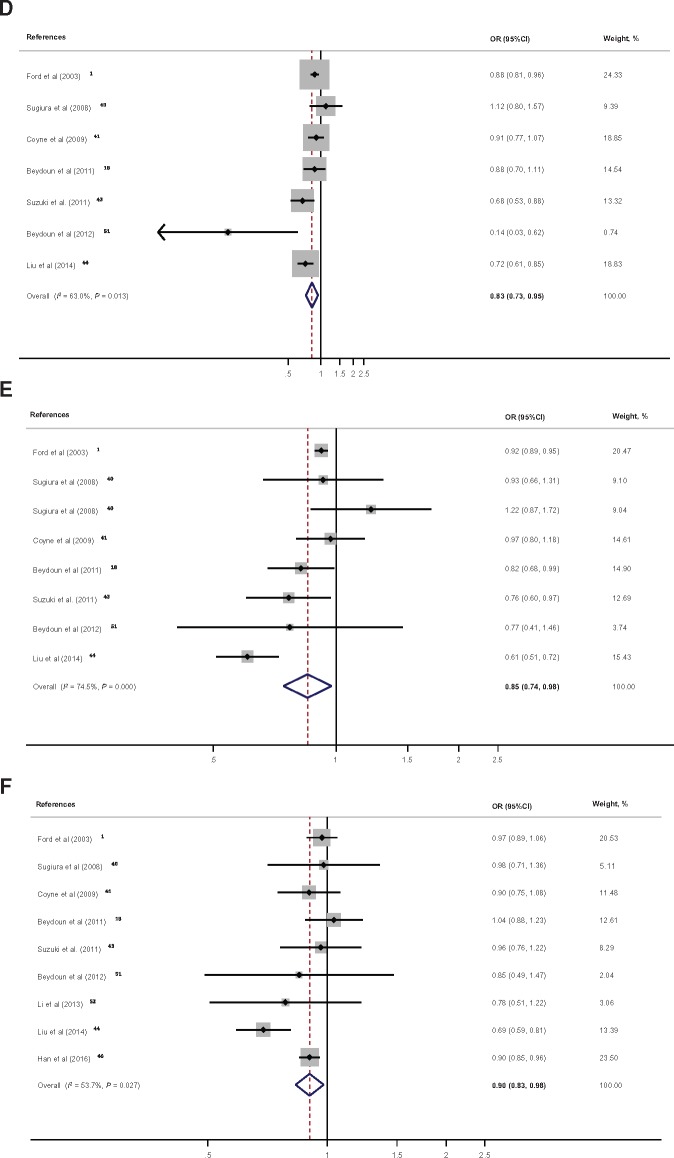
In total, measures of association from 11 studies on serum total and/or individual carotenoids in relation to MetS were pooled. Figure 3A shows a clear net inverse association between serum total carotenoids and MetS, with a pooled OR of 0.66 (95%CI, 0.56–0.78; 1 SD ∼ 0.82 µmol/L change; n = 5 studies; I2 = 76.2%) (see Figure S1C in the Supporting Information online for Cohen’s D pooled effect size). This inverse association was also noted for all individual carotenoids, with β-carotene showing the strongest putative protective effect, followed by α-carotene and β-crypotoxanthin (Figure 3B–F).
Figure 3.
Forest plot of odds ratios with 95% confidence intervals of the association between serum total and individual carotenoids and the metabolic syndrome, 1997–2017. A, One standard deviation of total carotenoids was estimated on average at 0.82 µmol/L with a standard error of 0.17.1,18,41,44,51B, One standard deviation of α-carotene was estimated on average at 0.22 µmol/L with a standard error of 0.08.1,18,40,41,43,44,51C, One standard deviation of β-carotene was estimated on average at 0.38 µmol/L with a standard error of 0.07.1,18,40,41,43,44,51,52D, One standard deviation of β-cryptoxanthin was estimated on average at 0.19 µmol/L with a standard error of 0.04.1,18,40,41,43,44,51E, One standard deviation of lutein/zeaxanthin was estimated on average at 0.09 µmol/L with a standard error of 0.05.1,18,40,41,43–45 Note that for Sugiura et al (2008)40 the 2 datapoints are for lutein and zeaxanthin separately. F, One standard deviation of lycopene was estimated on average at 0.27 µmol/L with a standard error of 0.07.1,18,40,41,43,44,46,51,52
Begg’s funnel plot (Figure S2 in the Supporting Information online) indicated that most of the 55 data points fell within the expected confidence limits when plotting Loge(OR) against its SE. In particular, Begg’s test indicated no publication bias, with z = 0.12 and associated P value of 0.91, although Egger’s test indicated asymmetry whereby Loge(OR)s were inversely related to their SEs in terms of slope. Moreover, inverse relationships between exposures and outcomes tended to be found in studies with larger variability in exposure and/or smaller sample sizes. In terms of bias, most Loge(OR)s suggested an inverse relationship between exposure and outcome, as expected in most hypothesized associations.
Harvest plots examining each individual carotenoid exposure are presented in Figure S3A–F in the Supporting Information online, plotting qualitative findings (−1 = “inverse association,” 0 = “null association,” and 1 = “positive association”) against study-level QS.56,57 Based on the clustering of findings, an inverse association between serum β-carotene and MetS appears to yield the most convincing evidence, with a mean QS of 6.1 (range, 4–7) and a total of 8 data points.
DISCUSSION
To the authors’ knowledge, this is the first review and meta-analysis to comprehensively assess the relationship of serum retinol (vitamin A), retinyl esters, and carotenoids with the clustering of cardiometabolic risk factors known as the metabolic syndrome (MetS). Among the key findings of this meta-analysis is the strong inverse associations of serum total carotenoids and β-carotene with MetS occurrence; these associations yield the most convincing evidence that consuming a diet rich in mixed carotenoids may be beneficial to cardio-metabolic health. With no indication of publication bias, harvest plots suggested the most consistency upon replication for serum β-carotene exposure.
Emerging evidence suggests that there is much to be learned about carotenoids and their conversion products in regards to a specific relationship with cardiometabolic risk factors.71 This meta-analysis synthesized some of the key related findings in the literature, indicating that serum total carotenoids, as well as β-carotene, may be cardioprotective. The failure of recent randomized control trials to show a beneficial effect of β-carotene supplements by themselves in preventing MetS may be explained in part by the fact that multiple antioxidants contained in natural fruits and vegetables may interact to increase the level of each carotenoid in serum. In fact, a previous study indicated that total antioxidant capacity was an independent predictor of β-carotene level.72 Although retinol (vitamin A) was previously found to have an inverse relationship with C-reactive protein, a measure of nonspecific inflammation often linked to poor cardiometabolic health,18,73 it was not associated with MetS itself based on this current meta-analysis.
Moreover, it is suggested that individuals with MetS have elevated oxidative stress markers, such as singleton oxygen and peroxyl molecules,74,75 leading to increased requirements for antioxidants, including pro-vitamin A carotenoids. Noteworthy is the large interindividual variation in the conversion of β-carotene to its retinoid metabolites, whereby 35%–75% of the absorbed β-carotene is converted to retinyl esters in the intestinal cells.76,77 Nevertheless, a similar inverse association was found between total carotenoids and retinyl esters with MetS, suggesting low exposure, mainly based on 2 large cross-sectional national studies of adults.1,18 Further longitudinal studies are needed to examine these relationships and ascertain temporality.
Despite limited data, this meta-analysis confirms a putative beneficial role of higher serum carotenoid in the regulation of the clustering of cardiometabolic risk factors associated with MetS. The role of retinol remains controversial and needs further inquiry. Although there are a host of factors that explain interindividual variability and carotenoid bioavailability in humans, these data suggest that carotenoids may play an essential role in adipose tissue biology, including the control of adipogenesis, oxidative stress, and the production of adipokines and inflammatory mediators that affect the distribution of central adiposity and the occurrence of insulin resistance.62,78–81 Because β-carotene intake in a typical Western diet can be as low as 1–5 mg/day from natural dietary sources, these data argue for establishing a recommended daily intake, which may be useful in preventing cardiometabolic disease.
This study has many strengths. First, this systematic review of the literature is, to the authors’ knowledge, one of few to have examined a wide range of vitamin A and carotenoid biomarkers and their relationships with MetS through meta-analysis. Second, a validated quality scoring system was used as a tool to examine the proportion of positive versus negative results according to quality of data. However, the study results should be interpreted with caution in light of several limitations. First, specific key terms were used to perform the literature search, but a search for cross-references or unpublished studies (abstracts, conference papers, theses, and dissertations) was not done. Second, the meta-analytic part of the study included serum concentrations of vitamin A and carotenoids as key exposures, whereas the qualitative part only included the related dietary exposures. Third, evidence was mostly generated from observational studies, with a focus on cross-sectional studies for the meta-analysis, which precludes one’s ability to confirm causality. Fourth, the associations reported in this study may be confounded by other carotenoids, nutrients, and lifestyle factors that have been shown to affect the risk of MetS. Nevertheless, many of the larger cross-sectional studies that were included in this meta-analysis controlled for a wide array of potentially confounding factors (eg, Ford et al,1 Beydoun et al,18 and Beydoun et al51). Finally, publication bias cannot be ruled out as an explanation for these study results, and heterogeneity among studies was found in many of the pooled findings, potentially explained by variations in unmeasured genetic factors, among others.
CONCLUSION
In sum, it is clear from previous studies that oxidative stress is associated with incidence of T2D and cardiovascular mortality and morbidity.82 This meta-analysis summarized the accumulating evidence that a higher level of oxidative stress also accompanies obesity-related disorders, which may be the causative agent behind further complications related to MetS, including the development of atherosclerosis. Serum levels of carotenoids, particularly α- and β-carotene, as well as retinyl esters, were inversely associated with MetS, whereas no statistically significant net association was found between serum retinol and the occurrence of MetS in the general population. Moreover, future intervention studies and randomized controlled trials related to dietary and lifestyle changes must be conducted to assess the utility of modifying serum concentrations of antioxidants, especially carotenoids, given their suboptimal levels among adults in the United States with MetS, for the prevention of T2D and various cardiovascular endpoints. Thus, establishing an adequate recommended daily intake and desirable serum levels of these compounds may be beneficial in future studies.
Acknowledgments
The authors would like to thank Gregory A. Dore and Ola S. Rostant (National Institute on Aging, National Institutes of Health, Intramural Research Program) for their internal review of the manuscript.
Author contributions.
M.A.B. was responsible for conceptualization; literature search and review; data management; plan of analysis; statistical analysis; write-up of manuscript; and revision of manuscript. X.C. was responsible for literature search and review; write-up of parts of manuscript; and revision of manuscript. K.J. was responsible for literature search and review; write-up of parts of the manuscript; and revision of the manuscript. H.A.B. was responsible for plan of analysis, write-up of parts of the manuscript; and revision of the manuscript. A.B.Z. was responsible for plan of analysis; write-up of parts of the manuscript; and revision of manuscript. J.A.C. was responsible for conceptualization; plan of analysis; literature review; write-up of parts of the manuscript; and revision of the manuscript. All authors read and approved the final manuscript. A.B.Z. and J.A.C. are co-senior authors.
Funding.
This research was supported entirely by the Intramural Research Program of the National Institutes of Health, National Institute on Aging.
Declaration of interest.
The authors have no relevant interests to declare.
Supporting Information
The following Supporting Information is available through the online version of this article at the publisher’s website.
Appendix S1 PRISMA checklist.
Appendix S2 Quality score.
Figure S1A–1C Forest plot of Cohen’s D with 95%CI of the association between serum vitamin A (per SD of (A) retinol1,18,47,48,51,52 or (B) retinyl ester1,18,51) or (C) Total carotenoids1,18,41,44,51 and the metabolic syndrome, 1997–2017.
Figure S2 Begg’s funnel plot for Ln(OR) with pseudo 95% confidence limits: all datapoints included in the meta-analysis (n = 55), 1997–2017.
Figure S3 Harvest plot of quality scores (QS) by type of exposures and qualitative finding.
Supplementary Material
References
- 1. Ford ES, Mokdad AH, Giles WH, et al. The metabolic syndrome and antioxidant concentrations: findings from the Third National Health and Nutrition Examination Survey. Diabetes. 2003;52:2346–2352. [DOI] [PubMed] [Google Scholar]
- 2. Grundy SM. Hypertriglyceridemia, insulin resistance, and the metabolic syndrome. Am J Cardiol. 1999;83:25F–29F. [DOI] [PubMed] [Google Scholar]
- 3. Alberti KG, Zimmet PZ.. Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: diagnosis and classification of diabetes mellitus provisional report of a WHO consultation. Diabet Med. 1998;15:539–553. [DOI] [PubMed] [Google Scholar]
- 4. Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults. Executive summary of the third report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, And Treatment of High Blood Cholesterol In Adults (Adult Treatment Panel III). JAMA. 2001;285:2486–2497. [DOI] [PubMed] [Google Scholar]
- 5. Alberti KG, Zimmet P, Shaw J; IDF Epidemiology Task Force Consensus Group. The metabolic syndrome—a new worldwide definition. Lancet. 2005;366:1059–1062. [DOI] [PubMed] [Google Scholar]
- 6. Galassi A, Reynolds K, He J.. Metabolic syndrome and risk of cardiovascular disease: a meta-analysis. Am J Med. 2006;119:812–819. [DOI] [PubMed] [Google Scholar]
- 7. Alberti KG, Eckel RH, Grundy SM, et al. Harmonizing the metabolic syndrome: a joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation. 2009;120:1640–1645. [DOI] [PubMed] [Google Scholar]
- 8. Appels CW, Vandenbroucke JP.. Overweight, obesity, and mortality. N Engl J Med. 2006;355:2699; author reply 2700–2701. [DOI] [PubMed] [Google Scholar]
- 9. Bender R, Zeeb H, Schwarz M, et al. Causes of death in obesity: relevant increase in cardiovascular but not in all-cancer mortality. J Clin Epidemiol. 2006;59:1064–1071. [DOI] [PubMed] [Google Scholar]
- 10. Doig GS. Obesity-related excess mortality: what should we do now? Crit Care Med. 2004;32:1084–1085. [DOI] [PubMed] [Google Scholar]
- 11. Ferrucci L, Alley D.. Obesity, disability, and mortality: a puzzling link. Arch Intern Med. 2007;167:750–751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Solomon CG, Manson JE.. Obesity and mortality: a review of the epidemiologic data. Am J Clin Nutr. 1997;66:1044S–1050S. [DOI] [PubMed] [Google Scholar]
- 13. Stevens J. Obesity mortality in Africans-Americans. Nutr Rev. 2000;58:346–353. [DOI] [PubMed] [Google Scholar]
- 14. Colditz GA. Economic costs of obesity and inactivity. Med Sci Sports Exerc. 1999;31:S663–S667. [DOI] [PubMed] [Google Scholar]
- 15. Hill JO, Sallis JF, Peters JC.. Economic analysis of eating and physical activity: a next step for research and policy change. Am J Prev Med. 2004;27:111–116. [DOI] [PubMed] [Google Scholar]
- 16. Wolf AM, Colditz GA.. Current estimates of the economic cost of obesity in the United States. Obes Res. 1998;6:97–106. [DOI] [PubMed] [Google Scholar]
- 17. Ford ES, Giles WH, Dietz WH.. Prevalence of the metabolic syndrome among US adults: findings from the third National Health and Nutrition Examination Survey. JAMA. 2002;287:356–359. [DOI] [PubMed] [Google Scholar]
- 18. Beydoun MA, Shroff MR, Chen X, et al. Serum antioxidant status is associated with metabolic syndrome among U.S. adults in recent national surveys. J Nutr. 2011;141:903–913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Zimmermann MB, Aeberli I.. Dietary determinants of subclinical inflammation, dyslipidemia and components of the metabolic syndrome in overweight children: a review. Int J Obes (Lond). 2008;32(suppl 6): S11–S18. [DOI] [PubMed] [Google Scholar]
- 20. Dakshinamurti K. Vitamins and their derivatives in the prevention and treatment of metabolic syndrome diseases (diabetes). Can J Physiol Pharmacol. 2015;93:355–362. [DOI] [PubMed] [Google Scholar]
- 21. Leermakers ET, Darweesh SK, Baena CP, et al. The effects of lutein on cardiometabolic health across the life course: a systematic review and meta-analysis. Am J Clin Nutr. 2016;103:481–494. [DOI] [PubMed] [Google Scholar]
- 22. de Toro-Martin J, Arsenault BJ, Despres JP, et al. Precision nutrition: a review of personalized nutritional approaches for the prevention and management of metabolic syndrome. Nutrients. 2017;9:pii E913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Bonet ML, Canas JA, Ribot J, et al. Carotenoids and their conversion products in the control of adipocyte function, adiposity and obesity. Arch Biochem Biophys. 2015;572:112–125. [DOI] [PubMed] [Google Scholar]
- 24. Soory M. Relevance of nutritional antioxidants in metabolic syndrome, ageing and cancer: potential for therapeutic targeting. Infect Disord Drug Targets. 2009;9:400–414. [DOI] [PubMed] [Google Scholar]
- 25. Campbell DR, Gross MD, Martini MC, et al. Plasma carotenoids as biomarkers of vegetable and fruit intake. Cancer Epidemiol Biomarkers Prev. 1994;3:493–500. [PubMed] [Google Scholar]
- 26. Stryker WS, Kaplan LA, Stein EA, et al. The relation of diet, cigarette smoking, and alcohol consumption to plasma beta-carotene and alpha-tocopherol levels. Am J Epidemiol. 1988;127:283–296. [DOI] [PubMed] [Google Scholar]
- 27. Rao AV, Rao LG.. Carotenoids and human health. Pharmacol Res. 2007;55:207–216. [DOI] [PubMed] [Google Scholar]
- 28. Voutilainen S, Nurmi T, Mursu J, et al. Carotenoids and cardiovascular health. Am J Clin Nutr. 2006;83:1265–1271. [DOI] [PubMed] [Google Scholar]
- 29. Montonen J, Knekt P, Jarvinen R, et al. Dietary antioxidant intake and risk of type 2 diabetes. Diabetes Care. 2004;27:362–366. [DOI] [PubMed] [Google Scholar]
- 30. Coyne T, Ibiebele TI, Baade PD, et al. Diabetes mellitus and serum carotenoids: findings of a population-based study in Queensland, Australia. Am J Clin Nutr. 2005;82:685–693. [DOI] [PubMed] [Google Scholar]
- 31. Ford ES, Will JC, Bowman BA, et al. Diabetes mellitus and serum carotenoids: findings from the Third National Health and Nutrition Examination Survey. Am J Epidemiol. 1999;149:168–176. [DOI] [PubMed] [Google Scholar]
- 32. Hozawa A, Jacobs DR Jr, Steffes MW, et al. Associations of serum carotenoid concentrations with the development of diabetes and with insulin concentration: interaction with smoking: the Coronary Artery Risk Development in Young Adults (CARDIA) Study. Am J Epidemiol. 2006;163:929–937. [DOI] [PubMed] [Google Scholar]
- 33. Reunanen A, Knekt P, Aaran RK, Aromaa A.. Serum antioxidants and risk of non-insulin dependent diabetes mellitus. Eur J Clin Nutr. 1998;52:89–93. [DOI] [PubMed] [Google Scholar]
- 34. Kataja-Tuomola M, Sundell JR, Mannisto S, et al. Effect of alpha-tocopherol and beta-carotene supplementation on the incidence of type 2 diabetes. Diabetologia. 2008;51:47–53. [DOI] [PubMed] [Google Scholar]
- 35. Liu S, Ajani U, Chae C, et al. Long-term beta-carotene supplementation and risk of type 2 diabetes mellitus: a randomized controlled trial. JAMA. 1999;282:1073–1075. [DOI] [PubMed] [Google Scholar]
- 36. Wang L, Liu S, Pradhan AD, et al. Plasma lycopene, other carotenoids, and the risk of type 2 diabetes in women. Am J Epidemiol. 2006;164:576–585. [DOI] [PubMed] [Google Scholar]
- 37. Wang L, Liu S, Manson JE, et al. The consumption of lycopene and tomato-based food products is not associated with the risk of type 2 diabetes in women. J Nutr. 2006;136:620–625. [DOI] [PubMed] [Google Scholar]
- 38. Grune T, Lietz G, Palou A, et al. Beta-carotene is an important vitamin A source for humans. J Nutr. 2010;140:2268S–2285S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Khillan JS. Vitamin A/retinol and maintenance of pluripotency of stem cells. Nutrients. 2014;6:1209–1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Sugiura M, Nakamura M, Ogawa K, et al. Associations of serum carotenoid concentrations with the metabolic syndrome: interaction with smoking. Br J Nutr. 2008;100:1297–1306. [DOI] [PubMed] [Google Scholar]
- 41. Coyne T, Ibiebele TI, Baade PD, et al. Metabolic syndrome and serum carotenoids: findings of a cross-sectional study in Queensland, Australia. Br J Nutr. 2009;102:1668–1677. [DOI] [PubMed] [Google Scholar]
- 42. Czernichow S, Vergnaud AC, Galan P, et al. Effects of long-term antioxidant supplementation and association of serum antioxidant concentrations with risk of metabolic syndrome in adults. Am J Clin Nutr. 2009;90:329–335. [DOI] [PubMed] [Google Scholar]
- 43. Suzuki K, Ito Y, Inoue T, Hamajima N.. Inverse association of serum carotenoids with prevalence of metabolic syndrome among Japanese. Clin Nutr. 2011;30:369–375. [DOI] [PubMed] [Google Scholar]
- 44. Liu J, Shi WQ, Cao Y, et al. Higher serum carotenoid concentrations associated with a lower prevalence of the metabolic syndrome in middle-aged and elderly Chinese adults. Br J Nutr. 2014;112:2041–2048. [DOI] [PubMed] [Google Scholar]
- 45. Sugiura M, Nakamura M, Ogawa K, et al. High serum carotenoids associated with lower risk for the metabolic syndrome and its components among Japanese subjects: Mikkabi cohort study. Br J Nutr. 2015;114:1674–1682. [DOI] [PubMed] [Google Scholar]
- 46. Han GM, Soliman GA, Meza JL, et al. The influence of BMI on the association between serum lycopene and the metabolic syndrome. Br J Nutr. 2016;115:1292–1300. [DOI] [PubMed] [Google Scholar]
- 47. Sharma P, Mishra S, Ajmera P, et al. Oxidative stress in metabolic syndrome. Indian J Clin Biochem. 2005;20:145–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Suriyaprom K, Phonrat B, Satitvipawee P, et al. Homocysteine but not serum amyloid A, vitamin A and E related to increased risk of metabolic syndrome in post-menopausal Thai women. Int J Vitam Nutr Res. 2014;84:35–44. [DOI] [PubMed] [Google Scholar]
- 49. Godala MM, Materek-Kusmierkiewicz I, Moczulski D, et al. Lower plasma levels of antioxidant vitamins in patients with metabolic syndrome: a case control study. Adv Clin Exp Med. 2016;25:689–700. [DOI] [PubMed] [Google Scholar]
- 50. Molnar D, Decsi T, Koletzko B.. Reduced antioxidant status in obese children with multimetabolic syndrome. Int J Obes Relat Metab Disord. 2004;28:1197–1202. [DOI] [PubMed] [Google Scholar]
- 51. Beydoun MA, Canas JA, Beydoun HA, et al. Serum antioxidant concentrations and metabolic syndrome are associated among U.S. adolescents in recent national surveys. J Nutr. 2012;142:1693–1704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Li Y, Guo H, Wu M, Liu M.. Serum and dietary antioxidant status is associated with lower prevalence of the metabolic syndrome in a study in Shanghai, China. Asia Pac J Clin Nutr. 2013;22:60–68. [DOI] [PubMed] [Google Scholar]
- 53. Borenstein M, Hedges LV, Higgins JPT, et al. Introduction to Meta-Analysis. Chichester, UK: John Wiley & Sons; 2009. [Google Scholar]
- 54. Petitti DB. Statistical methods in meat-analysis In: Petitti DB, ed. Meta-Analysis, Decision Analysis, and Cost-Effectiveness Analysis, 2nd ed New York: Oxford University Press; 2000: 94–118. [Google Scholar]
- 55. Carter P, Gray LJ, Troughton J, et al. Fruit and vegetable intake and incidence of type 2 diabetes mellitus: systematic review and meta-analysis. BMJ. 2010;341:c4229.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Crowther M, Avenell A, MacLennan G, et al. A further use for the harvest plot: a novel method for the presentation of data synthesis. Res Synth Methods. 2011;2:79–83. [DOI] [PubMed] [Google Scholar]
- 57. Ogilvie D, Fayter D, Petticrew M, et al. The harvest plot: a method for synthesising evidence about the differential effects of interventions. BMC Med Res Methodol. 2008;8:8.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Egger M, Smith GD, Altman DG.. Systematic Reviews in Heatlh Care: Meta-Analysis in Context, 2nd ed London: BMJ Publishing Group; 2001. [Google Scholar]
- 59. Begg CB, Mazumdar M.. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50:1088–1101. [PubMed] [Google Scholar]
- 60. Egger M, Davey Smith G, Schneider M, et al. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Stata Corporation. Statistics/Data Analysis: Release 14.0 [computer program]. College Station, TX: Stata Corporation; 2015.
- 62. Canas JA, Damaso L, Altomare A, et al. Insulin resistance and adiposity in relation to serum beta-carotene levels. J Pediatr. 2012;161:58–64.e1-2. [DOI] [PubMed] [Google Scholar]
- 63. Lim HS, Shin EJ, Yeom JW, et al. Association between nutrient intake and metabolic syndrome in patients with colorectal cancer. Clin Nutr Res. 2017;6:38–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Godala M, Materek-Kusmierkiewicz I, Moczulski D, et al. Should antioxidant vitamin supplementation be applied in patients with metabolic syndrome? A case-control study. Prz Menopauzalny. 2016;15:32–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Wei J, Zeng C, Gong QY, et al. Associations between dietary antioxidant intake and metabolic syndrome. PLoS One. 2015;10:e0130876.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Park S, Ham JO, Lee BK.. Effects of total vitamin A, vitamin C, and fruit intake on risk for metabolic syndrome in Korean women and men. Nutrition. 2015;31:111–118. [DOI] [PubMed] [Google Scholar]
- 67. Ali R, Lee ET, Knehans AW, et al. Dietary intake among American Indians with metabolic syndrome—comparison to dietary recommendations: the balance study. Int J Health Nutr. 2013;4:33–45. [PMC free article] [PubMed] [Google Scholar]
- 68. O'Neil CE, Nicklas TA, Rampersaud GC, et al. 100% orange juice consumption is associated with better diet quality, improved nutrient adequacy, decreased risk for obesity, and improved biomarkers of health in adults: national Health and Nutrition Examination Survey, 2003–2006. Nutr J. 2012;11:107.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Amini M, Esmaillzadeh A, Shafaeizadeh S, et al. Relationship between major dietary patterns and metabolic syndrome among individuals with impaired glucose tolerance. Nutrition. 2010;26:986–992. [DOI] [PubMed] [Google Scholar]
- 70. Sluijs I, Beulens JW, Grobbee DE, et al. Dietary carotenoid intake is associated with lower prevalence of metabolic syndrome in middle-aged and elderly men. J Nutr. 2009;139:987–992. [DOI] [PubMed] [Google Scholar]
- 71. Sesso HD. Carotenoids and cardiovascular disease: what research gaps remain? Curr Opin Lipidol. 2006;17:11–16. [DOI] [PubMed] [Google Scholar]
- 72. Valtuena S, Del Rio D, Pellegrini N, et al. The total antioxidant capacity of the diet is an independent predictor of plasma beta-carotene. Eur J Clin Nutr. 2007;61:69–76. [DOI] [PubMed] [Google Scholar]
- 73. Chen CC, Wu JY, Chang CT, et al. Levels of retinol-binding protein 4 and uric acid in patients with type 2 diabetes mellitus. Metab Clin Exp. 2009;58:1812–1816. [DOI] [PubMed] [Google Scholar]
- 74. Park K, Gross M, Lee D-H, et al. Oxidative stress and insulin resistance. Diabetes Care. 2009;32:1302–1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Palmieri VO, Grattagliano I, Portincasa P, et al. Systemic oxidative alterations are associated with visceral adiposity and liver steatosis in patients with metabolic syndrome. J Nutr. 2006;136:3022–3026. [DOI] [PubMed] [Google Scholar]
- 76. During A, Harrison EH.. Mechanisms of provitamin A (carotenoid) and vitamin A (retinol) transport into and out of intestinal Caco-2 cells. J Lipid Res. 2007;48:2283–2294. [DOI] [PubMed] [Google Scholar]
- 77. Schreiber R, Taschler U, Preiss-Landl K, et al. Retinyl ester hydrolases and their roles in vitamin A homeostasis. Biochim Biophys Acta. 2012;1821:113–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Canas JA, Lochrie A, McGowan AG, et al. Effects of mixed carotenoids on adipokines and abdominal adiposity in children: a pilot study. J Clin Endocrinol Metab. 2017;102:1983–1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Bohn T, Desmarchelier C, Dragsted LO, et al. Host-related factors explaining interindividual variability of carotenoid bioavailability and tissue concentrations in humans. Mol Nutr Food Res. 2017;61:1–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Bonet ML, Canas JA, Ribot J, et al. Carotenoids in adipose tissue biology and obesity. Subcell Biochem. 2016;79:377–414. [DOI] [PubMed] [Google Scholar]
- 81. Canas JA, Damaso L, Hossain J, et al. Fatty acid binding proteins 4 and 5 in overweight prepubertal boys: effect of nutritional counselling and supplementation with an encapsulated fruit and vegetable juice concentrate. J Nutr Sci. 2015;4:e39.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Roberts CK, Sindhu KK.. Oxidative stress and metabolic syndrome. Life Sci. 2009;84:705–712. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.