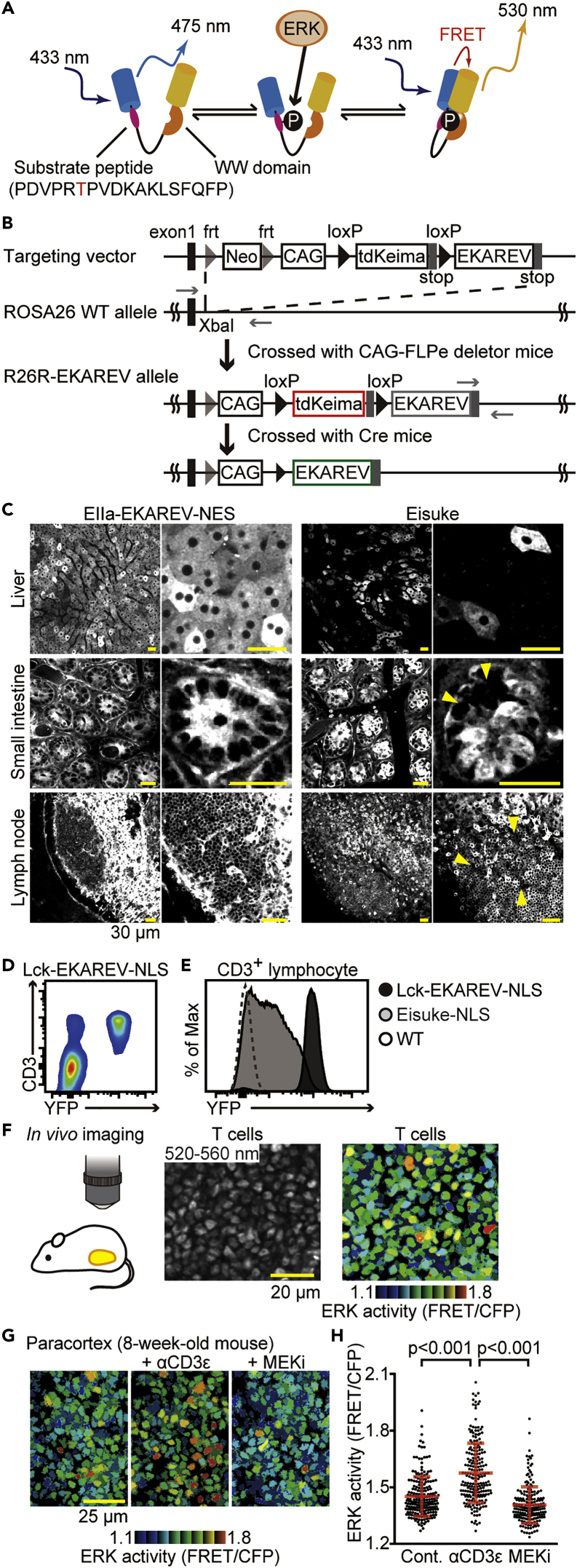
Figure 1.
Lck-EKAREV-NLS Mice Enable ERK Activity Monitoring in Lymphocytes
(A) A schema of EKAREV. Phosphorylation of the substrate peptide induces a conformational change and a concomitant increase in the FRET efficiency.
(B) A schema of the generation of R26R-EKAREV mice. Top to bottom: the structure of the targeting vector, the wild-type ROSA26 locus with the location of the insertion site, the structure of the R26R-EKAREV allele after flippase-frt-mediated excision of the frt-flanked neo cassette, and the structure of the R26-EKAREV allele after Cre-loxP-mediated excision of the loxP-flanked tdKeima sequence. Fragments shown in red and green can be expressed. The black rectangles on the left indicate the location of the first exon of the non-coding RNA in the ROSA26 locus. The gray rectangles indicate the location of the stop codons. loxP sequences are indicated by black arrowheads. frt sequences are indicated by gray arrowheads. Neo is the neo cassette. DT-A is a diphtheria toxin A fragment gene for negative selection.
(C) Representative fluorescence images of EIIa-EKAREV-NES (left) and Eisuke (right) through a BA 520-560 nm filter shown in grayscale. The excitation wavelength was 840 nm. Top to bottom: the liver, the small intestine, and the lymph node. Left to right: image of EKAREV fluorescence and enlarged view of the left image. The yellow arrowheads indicate the regions with the CAG promoter being inactive or only weakly active. Scale bar, 30 μm.
(D) Flow cytometric profile of EKAREV and CD3 expression among lymphocytes obtained from the lymph node of Lck-EKAREV-NLS. EKAREV expression is represented by YFP intensity.
(E) Flow cytometry of EKAREV-NLS expression in CD3+ lymphocytes of the lymph nodes derived from C57BL/6 (WT), Eisuke-NLS, and Lck-EKAREV-NLS mice.
(F) Images of the paracortex region of the lymph node in a living mouse obtained by TPEM as shown in the schema. (Left) Fluorescence image of T cells through a BA 520-560 nm emission filter. (Right) FRET/CFP ratio image shown in the intensity-modulated display (IMD) mode. Scale bar, 20 μm.
(G) Representative FRET/CFP ratio images of the T cells in the paracortex shown in IMD mode. Time-lapse imaging of T cells in the paracortex is performed for 90 min. Anti-CD3ɛ antibody (50 μg/body) was injected intravenously at 0 min. After 60 min, MEK inhibitor (PD0325901) (100 μg/body) was injected intravenously. The age of mouse in weeks is indicated. Left to right: FRET/CFP ratio image obtained just before anti-CD3ɛ antibody administration, 60 min after anti-CD3ɛ antibody administration, and 30 min after MEK inhibitor administration. Scale bar, 25 μm.
(H) The FRET/CFP ratio just before anti-CD3ɛ antibody administration (n = 211 cells), 60 min after anti-CD3ɛ antibody administration (n = 212 cells), and 30 min after MEK inhibitor administration (n = 207 cells). Similar experiments were performed with two independent mice and shown in Figure S1. Dots indicate the FRET/CFP ratio in each T cell. All data are presented as mean ± SD. p values were calculated by Student's two-sample t test.