Abstract
Background
Isavuconazole is a new antifungal prodrug for the treatment of invasive aspergillosis and mucormycosis. As no clear pharmacokinetic-pharmacodynamic relationship has been established for patients, therapeutic drug monitoring is not currently required. However, as isavuconazole is a new drug, clinicians are sometimes sceptical about the exposure achieved in their patients and seek pharmacokinetic exploration. A minimal response consists of determining that the patient’s pharmacokinetic profile agrees with profiles reported by Desai et al. using concentrations from the SECURE study.
Methods
Based on one concentration and Desai et al.’s population-pharmacokinetic model, it is possible to estimate a patient’s most likely pharmacokinetic profile. If a patient’s pharmacokinetic profile is close to the profiles reported by Desai et al., therapeutic drug monitoring is not required. In contrast, when the pharmacokinetic profile differs from the Desai et al. profiles, isavuconazole concentration monitoring and pharmacokinetic profile modeling are the only methods for obtaining information on a patient’s exposure and the efficacy of isavuconazole.
Results
Four patients presented with surprising pharmacokinetic profiles, unexplained by drug interactions or cytochrome P450 3A4/5 polymorphisms. For two of them, a drug dosage adjustment was proposed and applied by clinicians, together with a check for a new pharmacokinetic profile a few days later.
Conclusions
Collecting one blood sample just before the first maintenance dose to make an early estimation of the patient’s most likely pharmacokinetic profile is one method of identifying patients with outlier pharmacokinetic behavior.
Key Points
| Isavuconazole can require therapeutic drug monitoring. |
| We suggest collecting one blood sample just before the first maintenance dose to estimate the patient’s most likely kinetic profile using Desai et al.’s population-pharmacokinetic model. |
| If the kinetic profile is close to the profiles simulated with Desai et al.’s population-pharmacokinetic model, there is no need to perform therapeutic drug monitoring. |
| If the kinetic profile is outside the expected range, an individualized dose adjustment may be proposed (together with an estimation of the new kinetic profile). For these patients, we suggest screening cytochrome P450 3A4 and 3A5 genetic polymorphisms to explain these profiles. |
Introduction
Isavuconazole is a new antifungal prodrug approved in USA and Europe for the treatment of invasive aspergillosis and mucormycosis in adults. The recommended dose is 200 mg (intravenously or orally) every 8 h for 48 h, followed by 200 mg once daily as a maintenance dose applied 12–24 h after the last loading dose. Isavuconazole is mainly metabolized by the hepatic cytochrome 450 (CYP) 3A4/5 [1, 2], with polymorphisms [CYP3A4*22 (rs35599367) and CYP3A5*3 (rs776746)] that lead to unexpected kinetic profiles, as observed for other drugs such as tacrolimus [3] (an immunosuppressive drug). To date, the pharmacokinetic-pharmacodynamic relationship has not been established for patients [4], as the only information available was obtained using rabbits [5, 6] or a murine model [7, 8]. Consequently, a therapeutic range for patient exposure is not available and standard therapeutic drug monitoring (based, for example, on trough concentration as is often the case) is not currently recommended. However, as isavuconazole is a new drug, clinicians are sometimes sceptical about the exposures achieved among their patients and seek pharmacokinetic exploration. Currently, the only available information on pharmacokinetic parameters and their variability is provided by the population-pharmacokinetic (POP-PK) study published by Desai et al. [9] In their study, the authors proposed a validated POP-PK model that was created using patients’ concentrations collected from phases I and III of the SECURE trial [10]. In addition to the above-mentioned statements indicating that the pharmacokinetic-pharmacodynamic relationships have not yet been established for patients, in the SECURE trial, there was no difference between mortality or clinical success, whatever the measured exposures. Consequently, in the absence of any further information, in clinical practice, one recommendation could be at least to mimic the pharmacokinetic profiles reported in the SECURE trial.
Currrently, based on one concentration and Desai et al.’s POP-PK model, it is possible to estimate the most likely kinetic profile of a patient. If a patient’s kinetic profile is close to the profiles reported by Desai et al. [9], there is no need to perform therapeutic drug monitoring as recently suggested by Andes et al. [11] In contrast, when the kinetic profile differs from the Desai et al.’s profiles, isavuconazole concentration monitoring and kinetic profile modeling are the only methods for obtaining information on a patient’s exposure and the efficacy of isavuconazole. We used these methods for four patients with invasive aspergillosis who presented with unexpected high concentrations a few days after the last loading dose, without obvious explanatory factors (no drug–drug interactions as co-prescriptions do not inhibit CYP3A4/5 and no previous hepatic injury). For two patients, these high concentrations were concomitant with adverse effects (patellar tendinopathy and discomfort, respectively) that disappeared during the dose change or interruption. However, to date, it is not clear if there is a causal link.
Methods
We applied this approach to four patients who received oral isavuconazole, i.e., 200 mg every 8 h six times and then 200 mg every 24 h. As the patients were in a hospital and cared for by a specialized medical team, lack of compliance was unlikely. Isavuconazole plasma concentrations were determined using a validated chromatographic technique coupled with a diode array detector, according to European Medicines Agency, US Food and Drug Administration, and ISO15189 guidelines. The lower limit of quantification was 0.2 µg/mL and the inter-assay variability was < 7%. Using the POP-PK model published by Desai et al. [9], we first simulated 50,000 pharmacokinetic profiles of isavuconazole for the recommended loading (200 mg/8 h six times) and maintenance doses (200 mg/day). Then, for each patient, we computed the empirical Bayes estimates of the pharmacokinetic parameters (Table 1) and deduced the most likely kinetic profile (Fig. 1). Because these patients were extremes with respect to Desai et al.’s model, we computed their empirical Bayes estimates using an individual parameter distribution with heavier tails than the classical multi-dimensional log-normal distribution.
Table 1.
Pharmacokinetic-pharmacogenetic exploration of isavuconazole in four French patients presenting with high unexpected plasma concentrations: this exploration was performed as part of the patients’ hospital care
| Patient | Clinical information | Pharmacogenetics | Pharmacokinetic parameters | Individualized dose adjustment | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sex | Age, years | Ethnic group | BMI (kg/m2) | Co-prescription | Adverse effects | CYP3A4*1 CYP3A5*3 | Clearance (L/h) | Distribution volume (L) | Half-life (h) | New dose (mg/24 h) | TDM | |
| 1 | Male | 70 | Caucasian | 20.8 | Methotrexate Prednisone Esomeprazole |
Patellar tendinopathy | CYP3A4: 1*/1* CYP3A5: 3*/3* |
0.62 | 120.23 | 190.01 | 100 | |
| 2 | Female | 52 | Caucasian | 20.2 | Prednisone | Discomfort | CYP3A4: 1*/22* CYP3A5: 3*/3* |
1.09 | 196.77 | 161.28 | 100 | |
| 3 | Male | 38 | Caucasian | Prednisone | CYP3A4: 1*/1* CYP3A5: 3*/3* |
1.41 | 88.14 | 69.00 | 100 | 2.22 µg/mL measured for 2.54 µg/mL estimated 2.55 µg/mL measured for 2.57 µg/mL estimated |
||
| 4 | Female | 50 | Caucasian | Levothyrox Oxazepam Zopiclone Clonazepam |
CYP3A4: 1*/22* CYP3A5: 3*/3* |
1.03 | 141.23 | 134.40 | 100 | 1.83 µg/mL measured for 2.09 µg/mL estimated | ||
BMI body mass index, CYP cytochrome P450, TDM therapeutic drug monitoring
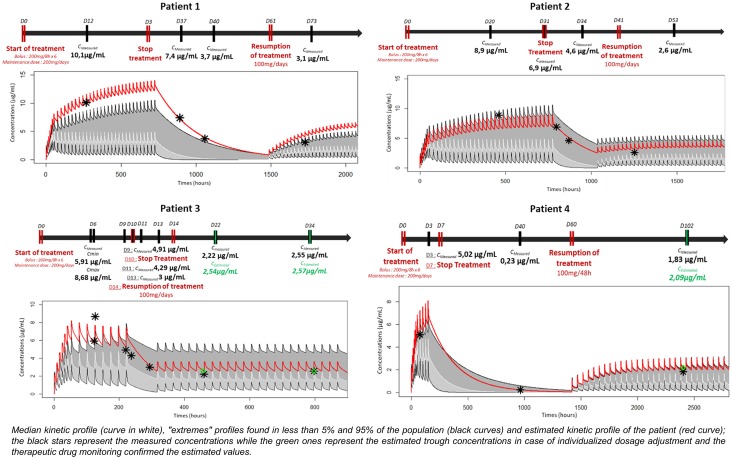
Fig. 1.
Patients 1–4: kinetic profile of isavuconazole. The white curve shows the median kinetic profile. “Extremes” profiles were found in less than 5% and greater than 95% of the population (black curves). The red curve shows the estimated kinetic profile of the patient. The black stars represent the measured concentrations while the green stars represent the estimated trough concentrations in the case of individualized dosage adjustment. Therapeutic drug monitoring confirmed the estimated values. Cestimated, estimated concentration using Desai's POP PK model, Cmax maximum plasma concentration, Cmeasured, measured concentration from the patient's blood sample D day
As isavuconazole is mainly metabolized by hepatic CYP3A4 and 3A5 [1, 2], we performed a pharmacogenetic exploration of CYP3A4*22 (rs35599367) and CYP3A5*3 (rs776746) polymorphisms using real-time polymerase chain reaction [12]. This pharmacokinetic-pharmacogenetic exploration was part of the hospital care for patients according to regulations applied in France (patient’s informed consent for genotyping).
Results
For all patients, the pharmacokinetic parameters differed from the median values (clearance 2.36 L/h, peripheral distribution volume 241.28 L, elimination half-life 90.57 h) obtained from 50,000 simulations performed with Desai et al.’s model [7] and, in particular, clearance was systematically lower (Table 1). With clinician guidance, an individualized dose adjustment was proposed for two patients (Fig. 1, Table 1) and therapeutic drug monitoring confirmed the estimated values were very close to the measured values (2.54 µg/mL and 2.57 µg/mL vs. 2.22 µg/mL and 2.55 µg/mL for patient 3 and 2.09 µg/mL vs. 1.83 µg/mL for patient 4).
The four patients all carried the homozygous variant CYP3A5*3, which is associated with a lack of CYP3A5 activity, while two patients were homozygous for CYP3A4*22. In the Caucasian population, close to Desai et al.’s study population (83.2% Caucasian and 16.8% Asian [12]), more than 80–90% of patients possessed the CYP3A5*3 polymorphism [13]. Consequently, this information cannot explain the over-exposure among our patients. On the contrary, the CYP3A4*22 polymorphism is present in less than 8–10% of the Caucasian population [14]. Only two patients (50% of our population, patients 2 and 4) presented with this polymorphism, suggesting that other explanatory factors are likely.
Conclusion
In current practice, it is implicitly assumed that the pharmacokinetic profile of a patient will be close to the pharmacokinetic profiles published by Desai et al. Unfortunately, without any therapeutic drug monitoring, there is no proof that this is true. In this respect, we suggest collecting one blood sample just before the first maintenance dose to provide an early estimation of the patient’s most likely kinetic profile using Desai et al.’s POP-PK model. If the kinetic profile is close to the profiles simulated with Desai et al.’s POP-PK model, there is no need for therapeutic drug monitoring. In contrast, if the kinetic profile is outside the expected range, an individualized dose adjustment may be proposed, together with an estimation of a new kinetic profile.
Although this approach has not been validated, given the limited number of patients and the lack of clinical data, obtaining a patient’s kinetic profile to fit the expected profiles is reassuring for clinicians. This is particularly true for specific populations such as patients with severe gut disease as a result of graft-vs.-host disease (for which oral drug absorption is problematic) and children, where there is sparse information on isavuconazole exposure [4].
We also recommend this approach when screening for CYP3A4 and 3A5 genetic polymorphisms, in addition to those usually explored (CYP3A4*22 and CYP3A5*3), including the non-functional alleles CYP3A4*17 (rs4987161) [15], CYP3A5*6 (rs10264272) [13], and CYP3A5*7 (rs76293380) [13] for patients presenting with unexpected kinetic profiles. We acknowledge that these suggestions are not applicable to all hospitals. One initial constraint is that most pharmacology laboratories do not have the ability to rapidly integrate trough serum data into a POP-PK model. The second constraint is that access to pharmacogenetics is limited in most settings. Finally, the use of CYP3A4/5 pharmacogenetic data is investigational and has not been validated for isavuconazole dosage adjustment. However, for patients in real life, there is scant information available on factors affecting pharmacokinetic profiles and pharmacogenetics is too interesting a path to be neglected.
Funding
The authors received no funding for this project: the data have been generated as part of routine work.
Conflict of interest
All authors have no conflicts of interest that are directly relevant to the contents of this article.
Contributor Information
Léa Darnaud, Phone: +33(0)567690383, Email: darnaud.lea@orange.fr.
Peggy Gandia, Phone: +33(0)567690383, Email: gandia.p@chu-toulouse.fr.
References
- 1.Amsden JR, Gubbins PO. Pharmacogenomics of triazole antifungal agents: implications for safety, tolerability and efficacy. Expert Opin Drug Metab Toxicol. 2017;13(11):1135–1146. doi: 10.1080/17425255.2017.1391213. [DOI] [PubMed] [Google Scholar]
- 2.Townsend R, Dietz A, Hale C, et al. Pharmacokinetic evaluation of CYP3A4-mediated drug-drug interaction of isavuconazole with rifampin, ketoconazole, midazolam, and ethinyl estradiol/norethindrone in healthy adults. Clin Pharmacol Drug Dev. 2017;6(10):44–53. doi: 10.1002/cpdd.285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pallet N, Jannot AS, El Bahri M, et al. Kidney transplant recipients carrying the CYP3A4*22 allelic variant have reduced tacrolimus clearance and often reach supratherapeutic tacrolimus concentrations. Am J Transplant. 2015;15(3):800–805. doi: 10.1111/ajt.13059. [DOI] [PubMed] [Google Scholar]
- 4.Desai A, Kovanda LL, Hope W, et al. Exposure-response relationships for isavuconazole in patients with invasive aspergillosis and other filamentous fungi. Antimicrob Agents Chemother. 2017;61(12):e01034-17. doi: 10.1128/AAC.01034-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kovanda LL, Petraitiene R, Petraitis V, et al. Pharmacodynamics of isavuconazole in experimental invasive pulmonary aspergillosis: implications for clinical breakpoints. J Antimicrob Chemother. 2016;71(7):1885–1891. doi: 10.1093/jac/dkw098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Petraitis V, Petraitiene R, Moradi PW, et al. Pharmacokinetics and concentration-dependent efficacy of isavuconazole for treatment of experimental invasive pulmonary aspergillosis. Antimicrob Agents Chemother. 2016;60(5):2718–2726. doi: 10.1128/AAC.02665-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lepak AJ, Marchillo K, Vanhecker J, Andes DR. Isavuconazole (BAL4815) pharmacodynamic target determination in an in vivo murine model of invasive pulmonary aspergillosis against wild-type and cyp51 mutant isolates of Aspergillus fumigatus. Antimicrob Agents Chemother. 2013;57(12):6284–6289. doi: 10.1128/AAC.01355-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Seyedmousavi S, Bruggemann RJ, Meis JF, et al. Pharmacodynamics of isavuconazole in an Aspergillus fumigatus mouse infection model. Antimicrob Agents Chemother. 2015;59:2855–2866. doi: 10.1128/AAC.04907-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Desai A, Kovanda LL, Kowalski D, et al. Population pharmacokinetics of isavuconazole from phase 1 and phase 3 (SECURE) trials in adults and target attainment in patients with invasive infections due to Aspergillus and other filamentous fungi. Antimicrob Agents Chemother. 2016;60:5483–5491. doi: 10.1128/AAC.02819-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Maertens JA, Raad II, Marr KA, et al. Isavuconazole versus voriconazole for primary treatment of invasive mould disease caused by Aspergillus and other filamentous fungi (SECURE): a phase 3, randomized-controlled, non-inferiority trial. Lancet. 2016;387(10020):760–769. doi: 10.1016/S0140-6736(15)01159-9. [DOI] [PubMed] [Google Scholar]
- 11.Andes D, Kovanda L, Desai A, et al. Isavuconazole concentration in real-world practice: consistency with results from clinical trials. Antimicrob Agents Chemother. 2018;62(7), pii: e00585–18. 10.1128/AAC.00585-18 [DOI] [PMC free article] [PubMed]
- 12.Gijsen VM, Schaik RH, Elens L, et al. CYP3A4*22 and CYP3A combined genotypes both correlate with tacrolimus disposition in pediatric heart transplant recipients. Pharmacogenomics. 2013;14(9):1027–1036. doi: 10.2217/pgs.13.80. [DOI] [PubMed] [Google Scholar]
- 13.Lamba J, Hebert JM, Schuetz EG, et al. PharmGKB summary: very important pharmacogene information for CYP3A5. Pharmacogenet Genomics. 2012;22(7):555–558. doi: 10.1097/FPC.0b013e328351d47f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Elens L, van Gelder T, Hesselink DA, et al. CYP3A4*22: promising newly identified CYP3A4 variant allele for personalizing pharmacotherapy. Pharmacogenomics. 2013;14(1):47–62. doi: 10.2217/pgs.12.187. [DOI] [PubMed] [Google Scholar]
- 15.Dai D, Tang J, Rose R, et al. Identification of variants of CYP3A4 and characterization of their abilities to metabolize testosterone and chlorpyrifos. J Pharmacol Exp Ther. 2001;299(3):825–831. [PubMed] [Google Scholar]