Abstract
Background
Progressive supranuclear palsy (PSP) is a neuropathologically defined disease with a broad clinical spectrum. It can initially be mistaken for other neurodegenerative diseases. Diagnosis of PSP earlier in the course may reduce its psychological and financial burden, permit earlier access to neuroprotective interventions, and avoid unnecessary diagnostic and therapeutic measures. Our impression is that physicians are more aware of PSP in the 2010s than in the 1990s. This study tests that hypothesis using the latency from symptom onset to PSP diagnosis as a surrogate outcome.
Methods
We reviewed records of 385 patients with “possible” or “probable” PSP from 1990 to 2016 at the Movement Disorders Center, Rutgers Robert Wood Johnson Medical School. The time from symptom onset to diagnosis was calculated for each patient and labeled as latency. We used the Pearson correlation coefficient, Student's t‐test, and ANOVA as appropriate.
Results
Our data show that the mean latency (SD) from symptom onset to diagnosis PSP, in months, was 43.76 (25.60) in the 1990s, 40.76 (28.73) in the 2000s, and 29.15 (16.80) in the 2010s (P < .001). There was also an inverse relationship between age at onset and latency (Pearson's r = −0.23, P < .001). This relationship did not affect the statistical significance of our main observation.
Conclusion
Our finding suggests that there is a progressive reduction in the latency over the past three decades. It may reflect increased awareness of PSP by physicians in our referral area.
Keywords: diagnosis, latency, progressive supranuclear palsy, symptom onset
Introduction
Progressive supranuclear palsy (PSP) was not described in the medical literature until 1963, probably because it was previously mistaken for other conditions. Even since the original, excellent clinicopathologic characterization of the disorder, most patients with PSP are initially misdiagnosed with PD (for those with bradykinetic onset); Alzheimer's disease or depression (behavioral onset); or vestibular, cerebrovascular, epileptic or hemodynamic disorders (falls as the onset). Latency from symptom onset to a correct diagnosis of PSP is typically estimated at 3–4 years, which is about halfway through the average disease course.1
Among neurologists, if not among non‐neurologists, there is a reason to hope that awareness of PSP may have improved over recent decades, including the establishment of a lay advocacy and information organization, CurePSP, in 1990 and the publication of autopsy‐validated diagnostic criteria in 1996.2 In order to provide patients and families with prognostic information and to spare them unnecessary diagnostic testing and fruitless therapeutic adventures, it is important to improve the diagnostic sensitivity of the medical community to PSP, and now that neuroprotective treatment trials have begun, to allow earlier access to possible disease‐altering treatments.
Therefore, we posed the hypothesis that the latency from PSP symptom onset to diagnosis has improved over the past three decades.
Methods
We reviewed records of all 406 PSP patients seen from 1990 to 2016 at the Movement Disorders Center, Rutgers Robert Wood Johnson Medical School. All patients met criteria for “possible” or “probable” PSP (Steele‐Richardson type), as defined by the NINDS‐SPSP Criteria of Litvan et al.2 Patients enrolled before 1996 were diagnosed based on the criteria described by Golbe et al. in 1988,3 which is recording the month and year of the first symptom. In retrospect, this was part of the progressive disease picture and consistent with the known natural history of PSP. For 21 patients, this information was not adequately recorded, leaving 385 patients for analysis. In some patients, an outside physician suspected or confirmed the diagnosis of PSP. When this occurred, we used the month and year of diagnosis as our outcome datum. For other patients, no suspicion appeared in available patient‐ and family‐provided records or patient history. When this occurred, we used the month and year of our initial PSP suspicion for the diagnosis date.
All patients were examined and their diagnoses confirmed by author LIG; however, in some patients, when another departmental movement disorder specialist initially suspected or diagnosed PSP, we used the date of that neurologist's initial suspicion.
When a precise month of onset was not available, but the patient's history stated “early” in the year, we assigned January; “late” in the year, December; and when no point in the year was specified, July. We recorded the data in an Excel spreadsheet and performed data analysis using SPSS statistical software. For each patient, we calculated descriptive statistics and latency as the number of months between the month/year of onset and the month/year of PSP diagnosis. We then calculated the Pearson correlation coefficient between the year of onset and the latency, and we calculated the differences among the decades using ANOVA and Student's t‐test.
We considered the possibility that as the population of our region ages, the average onset age of PSP may be increasing. This may explain any observed univariate observation of reduced latency in more recent decades. Other data1 show that patients with older PSP onset progress more quickly and the median age of the population of New Jersey was 34.4 years in 1990, 36.7 in 2000 and 39.0 in 2010.4 More rapid progression may reduce latency from onset to diagnosis; therefore, we corrected our analysis for PSP age of onset using regression analysis.
Results
For the 385 evaluable patients with PSP, the mean (SD) latency from onset to diagnosis in months was 38.96 (26.01), median 35 months, range 0–173, overall mean onset age (SD) was 67.74 (7.78) years, and 191 (49.6%) of the patients were female.
Fig. 1 is a scatterplot comparing latency with onset year. There is a progressive reduction in the latency (SD) over the time span examined, 43.76 (25.63) months in the 1990s to 29.15 (16.80) months in the 2010s, a reduction of 33.4% (Fig. 1 and Table 1). This effect was significant at the P < .001 level. The change in latency from the 1990s to the 2000s averaged 3.29 months, while the change from the 2000s to the 2010s averaged 11.32 months. The latter was statistically significant at P < .001.
Figure 1.
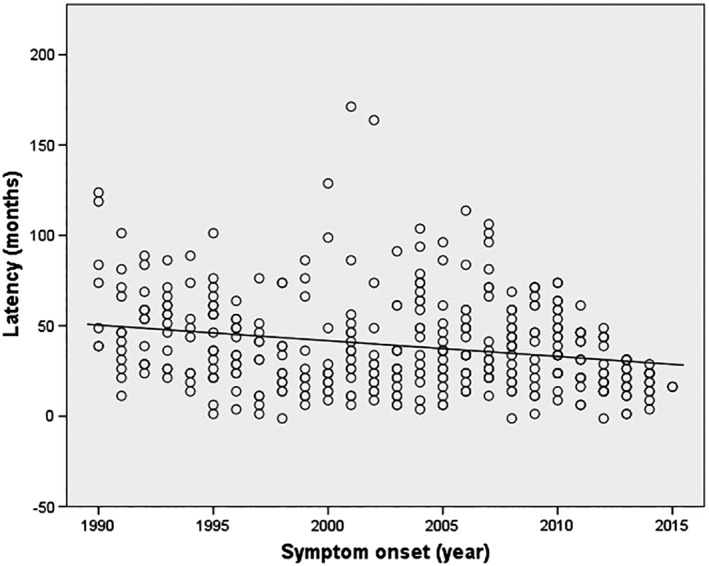
Scatterplot of latency (months) vs. onset year demonstrating a progressive improvement in latency from 1990 to 2015
Table 1.
Mean latency (months) from symptom onset to diagnosis of PSP over the past three decades
| Decades | N | Mean ± SD (range) | 95% CI |
|---|---|---|---|
| 1990s | 122 | 43.76 ± 25.63 (0–126) | 39.17–48.36 |
| 2000s | 176 | 40.47 ± 28.73 (0–173) | 36.20–44.75 |
| 2010s | 87 | 29.15 ± 16.80 (0–73) | 25.57–32.73 |
| Total | 385 | 38.96 ± 26.01 (0–173) | 36.36–41.56 |
Abbreviations: CI, confidence interval; N, number of patients; PSP, progressive supranuclear palsy; SD, standard deviation.
There was a significant relationship between latency and age of onset, with older patients having shorter latencies (Pearson's r = −0.23, P < .001; Fig. 2).
Figure 2.
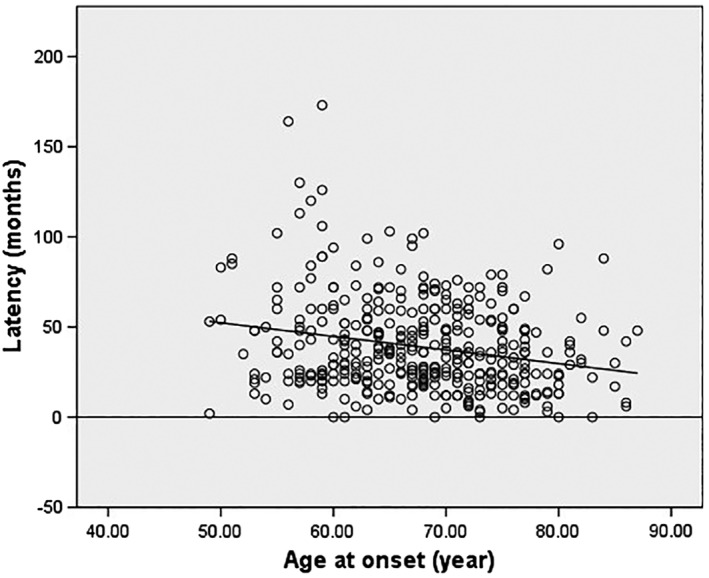
Scatterplot of latency (months) vs. age at onset (year) revealing a reduction in latency to diagnosis of PSP in older patients
The mean age of onset of the three groups examined (the 1990s, 2000s, and 2010s) showed an insignificant upward trend (P = 0.42). Linear regression analysis showed that the relationships between latency and onset year and between latency and onset age were mutually independent. For onset age, the odds ratio was 0.50 (95% CI 0.36–0.68, P < .001). For onset year, the OR was 0.45 (95% CI 0.31–0.65, P < .001).
We considered the possibility that our results may be explained by an ascertainment bias caused by a relative predominance early in the time span of “probable” PSP as opposed to “possible” PSP by the NINDS‐SPSP Criteria.2 Atypical forms of PSP may be more likely to satisfy only the “possible” criteria and to progress more slowly, producing a longer latency from onset to diagnosis. Therefore, we reviewed history and examination data from the initial visit for each of the first 20 patients seen in our cohort for each successive 5‐year interval, finding the following possible/probable ratios: 1994–1995: 7/13; 1999–2000: 8/12; 2004–2005: 7/13; 2009–2010: 10/10; and 2014–2015: 11/9. We conclude that there was no progressive reduction in the “possible” fraction in our cohort.
Discussion
We observed a moderate improvement in the latency to a diagnosis of PSP, from approximately 3.6 years in the 1990s to approximately 2.4 years in the 2010s, a reduction of 15 months, or 33%. Most of the observed reduction occurred from the 2000s to 2010s. Several factors may explain our observations. The first set of diagnostic criteria were published in 19883 and may have facilitated diagnosis from that point forward. The Society for Progressive Supranuclear Palsy, now called CurePSP, was founded in 1990 and has endeavored to improve awareness of the disease among those in the profession and the public. The NINDS‐SPSP Criteria were published in 1996 and may have facilitated clinical diagnosis. The celebrity Dudley Moore publicly announced his diagnosis of PSP in 1998 and died in 2002. These events probably increased awareness of PSP. Finally, the advent of the Internet in the 1990s may have improved awareness of rare diseases and of our center as a PSP referral center.
MRI came into widespread use in the 1980s, allowing neurologists to exclude such competing diagnostic considerations as vascular states and normal pressure hydrocephalus.5 However, it probably cannot explain the observed changes in diagnostic latency between the 1990s and subsequent decades.
The number of movement disorders specialists in New Jersey has increased dramatically over the observed timespan, from only one in 1980 to 14 today. This increase may help explain the decreasing latency that we observed over the period since 1990.
We considered the possibility that aging of the underlying population may explain our result, as the latency does have an inverse correlation with onset age in our subjects. However, our linear regression analysis found that the relationships between latency and onset age, and between latency and onset year were independent.
Failure of a patient with motor parkinsonism to respond to levodopa is often the first hint of a diagnosis of PSP. However, that drug came into use in the US in the early 1970s and would not explain our data.
Regardless of the mechanism underlying our observation, we can conclude that diagnostic care for PSP has improved markedly, at least in relatively wealthy and densely populated New Jersey. Further analysis of the reasons for the current latency averaging 2 years and 5 months may allow that figure to be pared further. Another reason for optimism is the recent recognition of minority phenotypes and presentations of PSP. For example, a patient presenting with parkinsonism typical for PD except for levodopa resistance, or one presenting with isolated gait apraxia, would now be considered a PSP candidate. That would not have been the case a few years ago.6, 7
Another observation here is that older symptom‐onset correlates with shorter latency between onset and diagnosis. A previous publication,1 using some of the present patients, showed that older onset age is a mortality risk in PSP. It seems likely that such patients’ more rapid progression facilitated speedier diagnosis after onset, despite the presence of diagnostic confounders in the elderly, such as orthopedic or cerebrovascular issues that might delay suspicion of a diagnosis of PSP.
Earlier diagnosis of PSP may improve the likelihood of patients responding to a neuroprotective treatment, several of which have entered clinical trials in recent years. Earlier diagnosis may also provide the patient and family with useful prognostic information, may confer a psychological benefit of knowing one's diagnosis, permit more participation in observational research, and allow patients and families to initiate support of, and participation in, PSP‐related organizations at an earlier stage of the illness.
Author Roles
1. Research project: A. Conception, B. Organization, C. Execution; 2. Statistical Analysis: A. Design, B. Execution, C. Review and Critique; 3. Manuscript Preparation: A. Writing of the first draft, B. Review and Critique.
M.M.: 1B, 1C, 3A.
H.R.: 2A, 2B, 2C, 3A.
L.I.G.: 1A, 1B, 2C, 3A, 3B.
Disclosures
Ethical Compliance Statement: We confirm that we have read the Journal's position on issues involved in ethical publication and affirm that this work is consistent with those guidelines. The authors confirm that the approval of an institutional review board/patient consent was not required for this work.
Funding Sources and Conflict of Interest: Supported by an Advanced Research Center of Excellence Grant from the American Parkinson's Disease Association and by the Movement Disorder Research Fund of Rutgers.
Financial Disclosures for the previous 12 months: The authors declare that there are no additional disclosures to report.
Relevant disclosures and conflicts of interest are listed at the end of this article.
Correction added on November 29, 2018, after first online publication: On page 2 in the last paragraph of the Methods section, the phrase “median population of individuals with PSP onset in New Jersey” was changed to “median age of the population of New Jersey”.
References
- 1. Golbe LI, Ohman‐Strickland PA. A clinical rating scale for progressive supranuclear palsy. Brain 2007; 130:1552–1565. [DOI] [PubMed] [Google Scholar]
- 2. Litvan I, Agid Y, Calne D et al Clinical research criteria for the diagnosis of progressive supranuclear palsy (Steele‐Richardson‐Olszewski syndrome): report of the NINDS‐SPSP International Workshop. Neurology 1996; 47:1–9. [DOI] [PubMed] [Google Scholar]
- 3. Golbe LI, Davis PH, Schoenberg BS et al Prevalence and natural history of progressive supranuclear palsy. Neurology 1988; 38:1031–1034. [DOI] [PubMed] [Google Scholar]
- 4. Unites States Census Bureau . 1990, 2000, and 2010 Census of Population: General Population Characteristics. Available at: http://www.census.gov/library/publications. Accessed June 18, 2018.
- 5. Whitwell JL, Höglinger JL, Bordelon Y et al Radiological biomarkers for diagnosis in progressive supranuclear palsy: Where are we and where do we need to be? Mov Disord 2017; 32:955–971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Höglinger GU, Respondek G, Stamelou M et al Clinical diagnosis of progressive supranuclear palsy: The movement disorder society criteria. Mov Disord 2017; 332:853–864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Respondek G, Stamelou M, Kurz C et al The phenotypic spectrum of progressive supranuclear palsy: a retrospective multicenter study of 100 definite cases. Mov Disord 2014; 29(14):1758–1766. [DOI] [PubMed] [Google Scholar]


