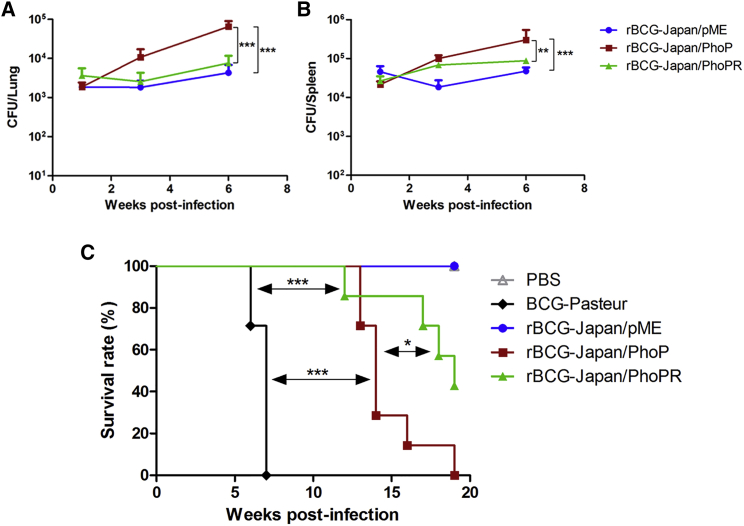
Figure 2.
Safety Profile of rBCG-Japan/PhoPR in SCID Mice
(A and B) SCID mice (n = 16 per group) were infected intravenously with 105 CFUs of the recombinant BCG-Japan strains (rBCG-Japan/PhoP and rBCG-Japan/PhoPR) or rBCG-Japan/pME as the control. At day 1 post-infection, four mice from each group were sacrificed to assess the initial infection doses, which were 2,677 ± 1,028, 2,080 ± 889, and 2,765 ± 965 CFUs/lung (mean ± SD) for rBCG-Japan/pME, rBCG-Japan/PhoP, and rBCG-Japan/PhoPR, respectively. At weeks 1, 3, and 6 post-infection, mice (n = 4 per group) were euthanized and bacterial burdens in the lungs (A) and spleen (B) were determined. Data are shown as mean ± SD (n = 4 SCID mice). Two-way ANOVA and Bonferroni multiple comparison tests were performed. (C) SCID mice (n = 10 per group) were infected intravenously with 107 CFUs of the indicated BCG strains. Three mice from each group were euthanized at day 1 post-infection to assess infection doses, which were 19,833 ± 14,530, 25,558 ± 10,335, 22,125 ± 7,730, and 28,200 ± 14,418 CFUs/lung (mean ± SD) for BCG-Pasteur, rBCG-Japan/pME, rBCG-Japan/PhoP, and rBCG-Japan/PhoPR, respectively. The remaining animals (n = 7 per group) were monitored weekly until they reached a humane endpoint. Survival curves were plotted using the Kaplan-Meier method. Log rank test was performed to compare each pair of the survival curves. *p < 0.05; **p < 0.01; ***p < 0.001.