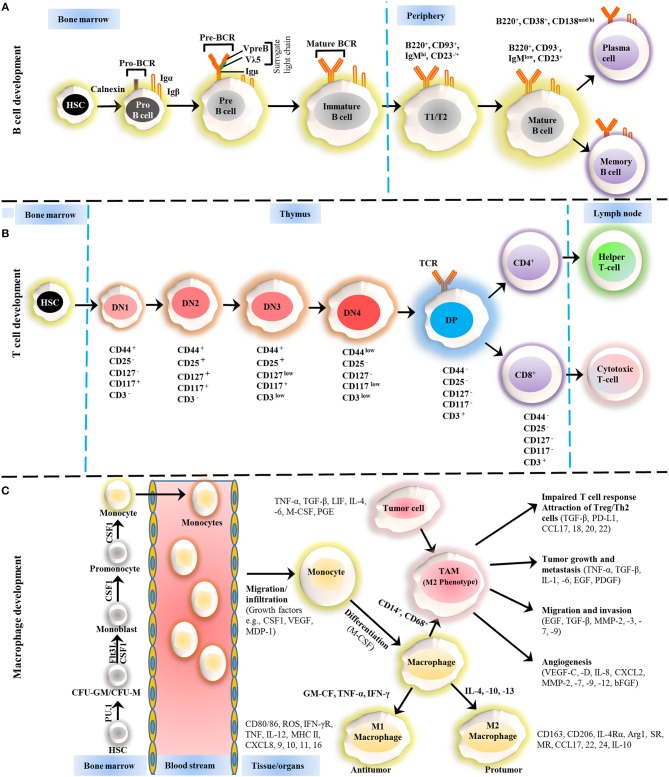
Figure 1.
Development of B cells, T cells and macrophages. (A) The development of B cells occurs in the bone marrow and peripheral lymphoid tissues. Development progresses from hematopoietic precursor cells (HSCs) and proceeds through several stages, such as a pro-B cell, pre-B cell, and immature B cell. During differentiation, the pre-B-cell receptor (pre-BCR) is generated following immunoglobulin locus rearrangements and is expressed on the cell surface. This pre-BCR (consisting of the surrogate light chain [VpreB or Vλ5] and an Igμ heavy chain) undergoes further rearrangements of the light and heavy-chain genes to form a mature BCR that can bind to the antigen. A selection process occurs at this immature B cell stage that prevents self-reactive cells from developing further. This stage is accompanied by both clonal selection and receptor editing. Those cells that successfully pass through this checkpoint (named transitional B cells) leave the BM and acquire their mature form as mature follicular B cells. (B) T cells development starts from HSCs in bone marrow and progresses to the thymus, where it passes through a series of developmental stages that can be recognized based on the expression of different cell surface markers. In the beginning of development, the expression of co-receptors CD4 and CD8 are absent and called double negative (DN) cells. The DN cells (DN1, DN2, DN3, and DN4) are further sub-divided by the expression of CD117, CD44, CD25, CD127, and CD3 markers. Further differentiation takes place by the up-regulation of CD4 and CD8 expression, therefore, names as double positive (DP) cells. The negative selection against self-antigen occurs in the thymus (medulla), where antigens are presented to them by dendritic cells and macrophages. T cells with stronger affinity then eliminated and the remaining T cells downregulate either co-receptor CD4 or CD8 and give rise to naïve cells stay in thymus and periphery. (C) The macrophage development and maturation also take place in bone marrow and tissues. From HSC, myeloid colony forming units are derived in bone marrow, and further grow into monocytes under colony-stimulating factor 1 (CSF1) through various highly organized stages. These monocytes can give rise to common DC progenitor cells that can transform into blood monocytes and, upon homing to various tissues except brain and skin macrophages, tissue macrophages. During most of the developmental stages, various factors influence the macrophage lineage development, however, CSF1 is likely imparted the highest influence. Finally, to address the inflammation, monocytes are recruited to tissues and restricted to specific phenotypes, M1, M2, and tumor-associated macrophages (TAM) depending upon inflammatory milieu. The development of TAM could also be influenced by tumor cells and tissue-resident macrophages. On the other hand, some other factors released by TAMs suppress the local immune response by either directly suppressing T cell responses or recruiting Treg cells. Arg1, arginase 1; bFGF, basic fibroblast growth factor; CCL, chemokine (C-C motif) ligand; CD, cluster of differentiation; CSF1 colony-stimulating factor-1; CXCL, chemokine (C-X-C motif) ligand; FLT3, FMS–like tyrosine kinase receptor 3; IFN, interferon; Ig, Immunoglobulin; IL, interleukin; LIF, leukocyte inhibitory factor; MDP, macrophage-derived proteoglycan; MHC, major histocompatibility complex; MMP, matrix metalloproteases; MR, Mineralocorticoid receptor; PD-L, Programmed death-ligand; PGE, prostaglandin; SR, scavenger receptor; TAM, tumor-associated macrophage; TGF, transforming growth factor; TNF, tumor necrosis factor; VEGF, vascular endothelial growth factor.