Abstract
Background
Intermittent left bundle branch block (LBBB) has been linked to chest pain, and causes cardiac memory electrocardiographic (ECG) changes mimicking ischemia.
Purpose
To present a case of chest pain with ECG abnormalities suggestive of ischemia, both likely caused by LBBB.
Case
A 33-year-old hypertensive female evaluated for chest pain and LBBB by ECG was treated with lisinopril and metoprolol, and scheduled for stress testing. A 12-lead ECG performed prior to the stress test, due to recurrence of the chest pain the preceding night, showed resolution of the LBBB with a lower heart rate, and T-wave inversions in the precordial leads suggestive of ischemia. She developed chest pains with reappearance of LBBB during stress testing, which prompted cardiac catheterization. This revealed normal coronaries and left ventricular systolic function. The ECG abnormalities were in retrospect likely due to cardiac memory. Her chest pains may have been caused by the intermittent, rate-related LBBB, as control of her heart rate and blood pressure with metoprolol and lisinopril improved her symptoms on follow-up.
Conclusion
Intermittent LBBB causes chest pain and electrocardiographic abnormalities suggestive of ischemia in the absence of obstructive coronary disease. Certain clinical and electrocardiographic features may provide clues to a non-ischemic etiology.
<Learning objective: In the absence of obstructive coronary disease, rate-related left bundle branch block is associated with chest pain described as local, non-radiating, with palpitations and walk-through phenomenon. It can also cause electrocardiographic (ECG) changes of cardiac memory, which mimic myocardial ischemia, but with T waves that are positive in lead aVL, positive or isoelectric in lead I, and more inverted in the precordial leads compared with lead III. These clinical and ECG features may provide clues to non-coronary etiology of chest pain.>
Keywords: Chest pain, Left bundle branch block, Cardiac memory, Electrocardiography
Introduction
Exercise-induced left bundle branch block (LBBB) has been described in 0.38% of patients referred for stress testing [1], and has been shown to be an independent predictor of a higher risk of death and major cardiac events [2]. Rate-related LBBB can happen spontaneously without exercise at any heart rate [3]. It has been linked to the development of angina-quality chest pain [4] and the appearance of abnormal, ischemic-type T wave inversions of cardiac memory [5], in the absence of obstructive coronary disease. A case that combines these features is presented, together with review of pertinent literature.
Case report
A 33-year-old female was referred to the cardiology clinic for evaluation of chest pain and palpitations. She reported intermittent left-sided chest discomfort radiating to her left arm. The pain was not always associated with exertional activities. Her symptoms would only last a few seconds and abate spontaneously. She also complained of dyspnea on moderate exertion and rare palpitations. She is a non-smoker and does not have family history of premature coronary artery disease. She was only taking oral contraceptive pills. Blood pressure was recorded as 170/110 mmHg with a regular heart rate of 100 bpm; the remainder of her physical examination was unremarkable. Electrocardiography (ECG) revealed sinus tachycardia with LBBB (Fig. 1), which was a new finding compared to a prior ECG seven years earlier (Fig. 2). Basic blood work revealed normal creatinine, electrolytes, glucose, thyroid stimulating hormone, and complete blood count.
Fig. 1.
Electrocardiogram upon presentation to cardiology clinic reveals sinus tachycardia at 103 bpm with left bundle branch block.
Fig. 2.
Baseline electrocardiogram, seven years earlier, reveals sinus rhythm with non-specific anterior T wave abnormalities.
The patient was started on lisinopril 20 mg daily and metoprolol tartrate 25 mg twice daily to treat her hypertension and palpitations. Given her symptoms and the newly diagnosed LBBB, she was scheduled for pharmacologic stress nuclear testing to evaluate any structural or functional abnormalities which may explain her chest discomfort, dyspnea on exertion, and palpitations.
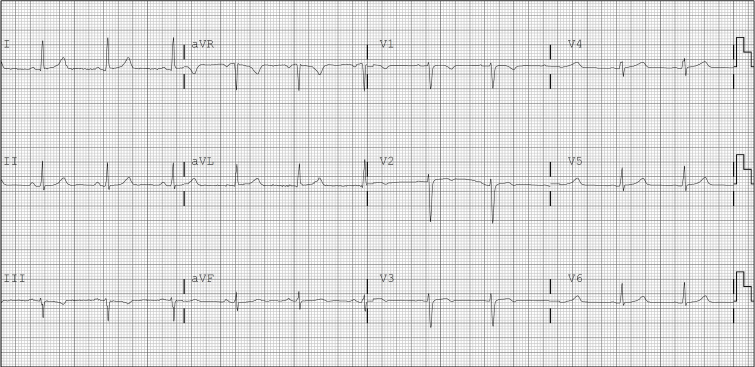
Two weeks later the patient presented for her scheduled tests. She reported one recurrent episode of chest discomfort the preceding evening. An ECG was obtained (Fig. 3) which revealed a slower heart rate of 64 bpm and resolution of the LBBB when compared with her clinic ECG (Fig. 1). T-wave inversions were noted in the precordial leads suggestive of ischemia, which were new compared to her previous baseline ECG (Fig. 2). Pharmacologic stress testing using regadenoson was performed, with re-emergence of the LBBB at 94 bpm, associated with her usual non-radiating, left-sided chest discomfort. Given the persistence of lingering chest discomfort while the patient was being observed after regadenoson was stopped, it was decided to proceed to coronary angiography. Left ventriculography was normal, with an estimated ejection fraction of 55%, and no mitral regurgitation. Coronary arteries were normal. Review of her pharmacologic stress test results revealed normal stress and rest myocardial perfusion with no evidence of ischemia; LVEF was 55%, in the normal range, and regional wall motion was normal. A follow-up visit three weeks later documented better blood pressure and heart rate control on metoprolol and lisinopril, with improved symptoms.
Fig. 3.
Electrocardiogram the day of stress test reveals sinus rhythm at 64 bpm with abnormal ST-T changes in the anterior leads suggestive of ischemia.
Discussion
Chatterjee et al. [6] described massive T wave inversion and ST depression in the unpaced ECG subsequent to ventricular pacing, which persisted for a varying length of time depending on the duration of pacing. Although similar findings of T wave inversions were subsequently reported with intermittent LBBB [7], it was not until 1982 when Rosenbaum et al. [8] coined the term “cardiac memory” to describe the phenomenon of reversible T-wave changes following abnormal ventricular rhythms. They illustrated alterations in the sinus T-waves that seemed to reflect the major vector of the QRS-complex during abnormal ventricular depolarizations. Abnormal ventricular rhythms that could predispose to these alterations are ventricular arrhythmias, ventricular pacing, and intermittent LBBB, as is the case in our patient.
The differential diagnosis of T-wave inversion is broad [9]; however, in the presence of chest pain, myocardial ischemia or infarction become highly suspected. De Zwaan et al. [10] demonstrated that patients who show characteristic ST-T segment changes in the precordial leads (commonly known as Wellens’ sign) on or shortly after admission, have a critical stenosis high in the left anterior descending coronary artery (LAD), and require urgent coronary angiography and, when possible, coronary revascularization. These ischemic changes share similarities with the precordial T wave abnormalities of cardiac memory; however, in cardiac memory the frontal leads usually remain unaffected except in lead III, where T wave inversion may be a normal variant, and in lead aVR. Shvilkin et. [11] proposed highly sensitive and specific criteria for T wave inversions which favor cardiac memory rather than ischemia (Table 1). In our case (Fig. 3), the T waves were positive in I and aVL, and the maximum T wave inversion in lead V2 exceeded the T wave inversion in lead III, thereby meeting all the criteria that favor cardiac memory over ischemia.
Table 1.
T-wave inversion criteria favoring cardiac memory rather than ischemia, proposed by Shvilkin et al. [11]. Combination of these findings yields 92% sensitivity and 100% specificity.
| 1. | Lead aVL: positive T-wave |
| 2. | Lead I: positive (or isoelecric) T-wave |
| 3. | Precordial leads: maximum T-wave inversion > T-wave inversion in Lead III |
Several cases of cardiac memory associated with LBBB in patients evaluated for chest pain have been reported [5], [12], [13], [14]. Whether the symptoms reported with cardiac memory were directly related to the ECG changes of cardiac memory, to the underlying depolarization abnormality leading to the cardiac memory, or were merely coincidental, remains unclear. Virtanen et al. [15] reported a triad of exertional chest discomfort, transient rate-dependent LBBB, and normal coronary arteries in patients with clinical symptoms resembling effort angina. They, however, noted that the symptom onset was always abrupt (simultaneously with the appearance of LBBB); the pain was local, never radiating; and that palpitation and “walk-through” phenomenon were often present (Table 2). Our patient exhibited some of those features during stress, as her pain was localized, abrupt, coinciding with the development of LBBB, and remained steady despite continuation of the stress test.
Table 2.
Characteristic non-anginal symptoms in a triad of exertional chest discomfort, transient rate-dependent LBBB, and normal coronary arteries, described by Virtanen et al. [15].
| 1. | Abrupt onset of pain was always abrupt (never gradually increasing during effort) |
| 2. | Localized pain was in the precordium or retrosternally (never radiated) |
| 3. | “Walk through” phenomenon was present |
| 4. | Palpitation in association with the chest pain was a common complaint |
No specific treatment is available for chest pain caused by rate-related LBBB. Heinsimer et al. [16] reported a 47-year-old female with chest pain associated with rate-related LBBB whose heart rate at onset of LBBB rose from 133 to 175 bpm after 3 months of exercise training; she no longer developed symptoms during routine daily activities or exercise. Shenoy and Sattur [5] reported a 48-year-old man with chest pain associated with rate-related LBBB whose symptoms resolved after starting a low-dose nonselective beta-blocker. Tanaka et al. [17] reported a 53-year-old male with nonischemic cardiomyopathy (EF 35%) and exercise-induced LBBB at 100 bpm; after 5 months of carvedilol and candesartan therapy for congestive heart failure, his ejection fraction improved to 49%, and the heart rate at which LBBB was induced increased to 126 bpm. Our patient's symptoms have improved after blood pressure and heart rate control with a combination of lisinopril and metoprolol.
The molecular mechanism of cardiac memory appears to involve the transient outward potassium current (Ito), the blockade of which suppresses cardiac memory [18], [19]. This current appears to be regulated by angiotensin II receptor-mediated signaling, which is activated by the mechanical stretch that results from the altered electrical activation of myocytes during pacing [20]. Therefore, cardiac memory has been suggested to portend the future development of hypertrophy [21], [22]. Angiotensin-converting enzyme inhibition and angiotensin receptor blockade prevent the development of cardiac memory in the canine heart [21], [23].
In summary, abnormal activation sequence of the myocardium especially by temporary pacing or transient LBBB, leads to abnormal T-waves suggestive of ischemia, usually confined to the precordial leads. When patients present with chest pain and such ischemic-looking T waves, it may be helpful to apply the criteria suggested by Virtanen et al. [15] with regards to the quality of the symptoms and the criteria proposed by Shvilkin et al. [11] in Table 1 with regards to the abnormal T waves to help sort out the etiology of the chest pain and plan further management.
Conflict of interest
Authors declare no conflict of interest.
References
- 1.Stein R., Ho M., Oliveira C., Ribeiro J., Lata K., Abella J., Olson H., Myers J. Exercise-induced left bundle branch block: prevalence and prognosis. Arq Bras Cardiol. 2011;97:26–32. doi: 10.1590/s0066-782x2011005000054. [DOI] [PubMed] [Google Scholar]
- 2.Grady T., Chiu A., Snader C., Marwick T., Thomas J., Pashkow F., Lauer M. Prognostic significance of exercise-induced left bundle-branch block. JAMA. 1998;279:153. doi: 10.1001/jama.279.2.153. [DOI] [PubMed] [Google Scholar]
- 3.Koito H., Spodick D. Physiologic differences in rate-related versus exercise-induced left bundle branch block. Am J Cardiol. 1988;62:316–319. doi: 10.1016/0002-9149(88)90235-4. [DOI] [PubMed] [Google Scholar]
- 4.Ninan M., Swan J. Can left bundle branch block cause chest pain. Br J Cardiol. 2002;9:230–232. [Google Scholar]
- 5.Shenoy C., Sattur S. The heart remembers. Am J Med. 2006;119:837–838. doi: 10.1016/j.amjmed.2006.08.013. [DOI] [PubMed] [Google Scholar]
- 6.Chatterjee K., Harris A., Davis G., Leatham A. Electrocardiographic changes subsequent to artificial ventricular depolarization. Br Heart J. 1969;31:770. doi: 10.1136/hrt.31.6.770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Denes P., Pick A., Miller R., Pietras R., Rosen K. A characteristic precordial repolarization abnormality with intermittent left bundle-branch block. Ann Intern Med. 1978;89:55–57. doi: 10.7326/0003-4819-89-1-55. [DOI] [PubMed] [Google Scholar]
- 8.Rosenbaum M., Blanco H., Elizari M., Lazzari J., Davidenk J. Electrotronic modulation of the T wave and cardiac memory. Am J Cardiol. 1982;50:213–222. doi: 10.1016/0002-9149(82)90169-2. [DOI] [PubMed] [Google Scholar]
- 9.Hayden G., Brady W., Perron A., Somers M., Mattu A. Electrocardiographic T-wave inversion: differential diagnosis in the chest pain patient. Am J Emerg Med. 2002;20:252–262. doi: 10.1053/ajem.2002.32629. [DOI] [PubMed] [Google Scholar]
- 10.de Zwaan C., Bär F., Wellens H. Characteristic electrocardiographic pattern indicating a critical stenosis high in left anterior descending coronary artery in patients admitted because of impending myocardial infarction. Am Heart J. 1982;103(4 Pt 2):730–736. doi: 10.1016/0002-8703(82)90480-x. [DOI] [PubMed] [Google Scholar]
- 11.Shvilkin A., Ho K., Rosen M., Josephson M. T-vector direction differentiates postpacing from ischemic T-wave inversion in precordial leads. Circulation. 2005;111:969–974. doi: 10.1161/01.CIR.0000156463.51021.07. [DOI] [PubMed] [Google Scholar]
- 12.Byrne R., Filippone L. Benign persistent T-wave inversion mimicking ischemia after left bundle-branch block—cardiac memory. Am J Emerg Med. 2010;28(747):e5–e6. doi: 10.1016/j.ajem.2009.10.003. [DOI] [PubMed] [Google Scholar]
- 13.Chapman J. Intermittent left bundle branch block in the athletic heart syndrome. Chest. 1977;71:776–779. doi: 10.1378/chest.71.6.776. [DOI] [PubMed] [Google Scholar]
- 14.Costantini M. Cardiac memory on Memorial Day: a nice coincidence. J Cardiovasc Med. 2012;13:160–161. doi: 10.2459/JCM.0b013e32834cd709. [DOI] [PubMed] [Google Scholar]
- 15.Virtanen K., Heikkilä J., Kala R., Siltanen P. Chest pain and rate-dependent left bundle branch block in patients with normal coronary arteriograms. Chest. 1982;81:326–331. doi: 10.1378/chest.81.3.326. [DOI] [PubMed] [Google Scholar]
- 16.Heinsimer J., Skelton T., Califf R. Rate-related left bundle branch block with chest pain and normal coronary arteriograms treated by exercise training. Am J Med Sci. 1986;292:317–319. doi: 10.1097/00000441-198611000-00013. [DOI] [PubMed] [Google Scholar]
- 17.Tanaka H., Hiraishi M., Miyoshi T., Tsuji T., Kaneko A., Ryo K., Yamawaki K., Fukuda Y., Morisada K., Tatsumi K., Matsumoto K., Kawai H., Hirata K. Exercise-induced left bundle branch block and subsequent mechanical left ventricular dyssynchrony—resolved with pharmacological therapy. Cardiovasc Ultrasound. 2011;9:4. doi: 10.1186/1476-7120-9-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Del Balzo U., Rosen M. T wave changes persisting after ventricular pacing in canine heart are altered by 4-aminopyridine but not by lidocaine. Circulation. 1992;85:1464–1472. doi: 10.1161/01.cir.85.4.1464. [DOI] [PubMed] [Google Scholar]
- 19.Geller J., Rosen M. Persistent T-wave changes after alteration of the ventricular activation sequence: new insights into cellular mechanisms of cardiac memory. Circulation. 1993;88:1811–1819. doi: 10.1161/01.cir.88.4.1811. [DOI] [PubMed] [Google Scholar]
- 20.Jeyaraj D., Ashwath M., Rosenbaum D. Pathophysiology and clinical implications of cardiac memory. Pacing Clin Electrophysiol. 2010;33:346–352. doi: 10.1111/j.1540-8159.2009.02630.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rosen M., Cohen I. Cardiac memory … new insights into molecular mechanisms. J Physiol. 2006;570(Pt 2):209–218. doi: 10.1113/jphysiol.2005.097873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rosen M., Cohen I., Danilo P., Steinberg S. The heart remembers. Cardiovasc Res. 1998;40:469–482. doi: 10.1016/s0008-6363(98)00208-9. [DOI] [PubMed] [Google Scholar]
- 23.Marrus S., Nerbonne J. Mechanisms linking short- and long-term electrical remodeling in the heart. Is it a stretch? Channels (Austin) 2008;2:117–124. doi: 10.4161/chan.2.2.6104. [DOI] [PubMed] [Google Scholar]