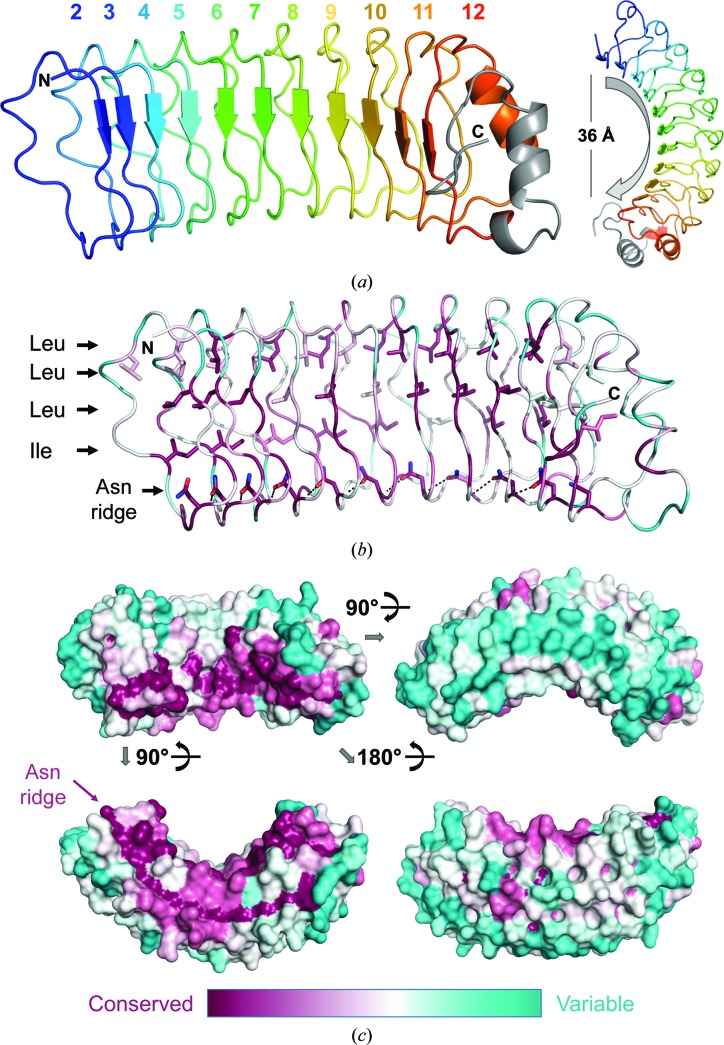
Figure 4.
SDS22 structure. (a) The structure of SDS22, with the LRRs indicated in distinct colors; the C-terminal cap is in gray. The horseshoe fold characteristic of LRR proteins is evident when viewing the molecule down the LRR β-strands (right). (b) An alignment of SDS22 sequences from 39 diverse species was used to identify the residue conservation throughout evolution (most conserved in dark pink to most divergent in teal) and map it onto the SDS22 surface. The majority of the conserved residues define the LRR fold (shown as sticks), including the hydrophobic core (Leu/Ile) and the Asn ridge. (c) Sequence conservation mapped onto the SDS22 surface; only the concave surface [upper left; the same orientation as in (a) and (b)] has highly conserved residues.