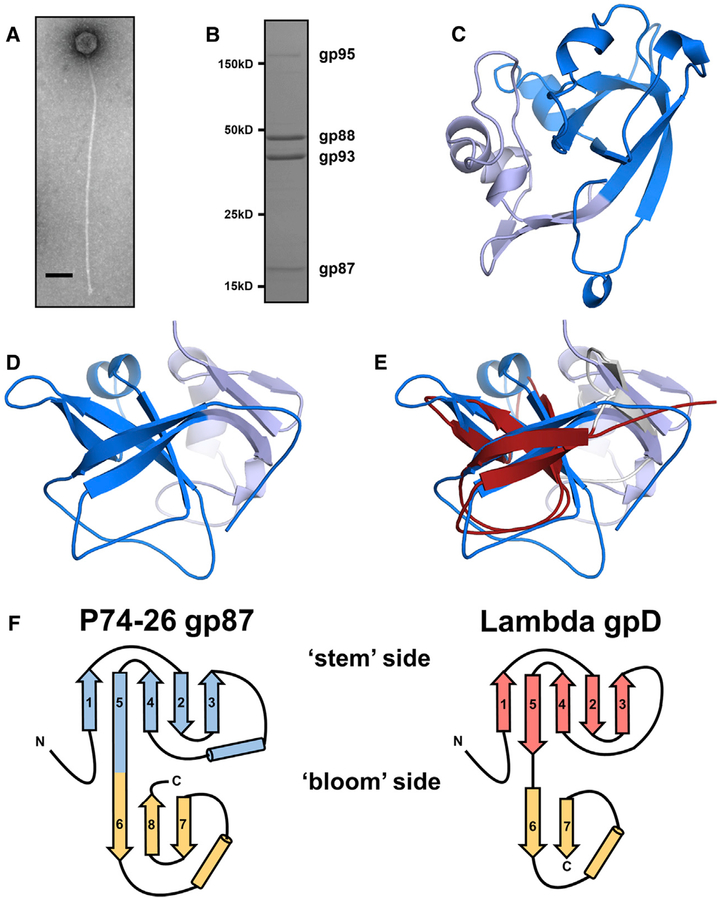
Figure 1. P74–26 gp87 Is a Thermophilic Capsid Decoration Protein.
(A) Negative-stain electron micrograph of purified P74–26 virion. Scale bar, 100 nm.
(B) SDS-PAGE analysis of P74–26 virions reveals major structural components including gp87, gp88 (MCP), gp93 (tail protein), and gp95 (tape measure protein).
(C and D) The 1.7-Å resolution structure of P74–26 gp87 with the five-stranded β tulip domain is highlighted in dark blue.
(E) Structure-based alignment of P74–26 gp87 (β tulip domain in blue) and λ decoration protein gpD (gray, β tulip domain in red, PDB: 1C5E) reveals significant structural homology despite high sequence variance.
(F) Topology diagrams of P74–26 gp87 and λ gpD reveal conserved architecture of β tulip domain flanked by a small mixed α/β domain.