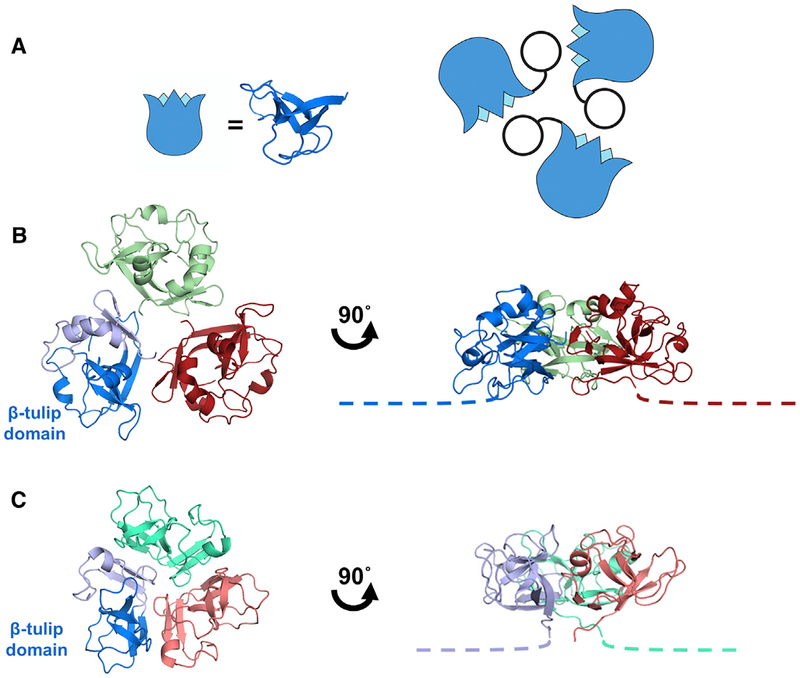
Figure 2. Interactions of the Decoration Protein for Capsid Stabilization.
(A) Model of decoration protein trimer highlighting positions of the β tulip domains (blue) within the assembly.
(B) P74–26 gp87 trimer highlights difference in trimer assembly characterized by a ~20° outward rotation of each of the gp87 trimer subunits (see also Video S2). The N-terminal capsid binding region of both crystal structures is disordered, and is drawn as proportional dotted lines in (B) and (C).
(C) Structure of the λ gpD trimer shows the orientation of gpD from the top of the capsid (left) and rotated 90° to the side (right).