Figure 3. Thermophilic Decoration Protein Has Enhanced Stability Compared with Mesophilic Homologs.
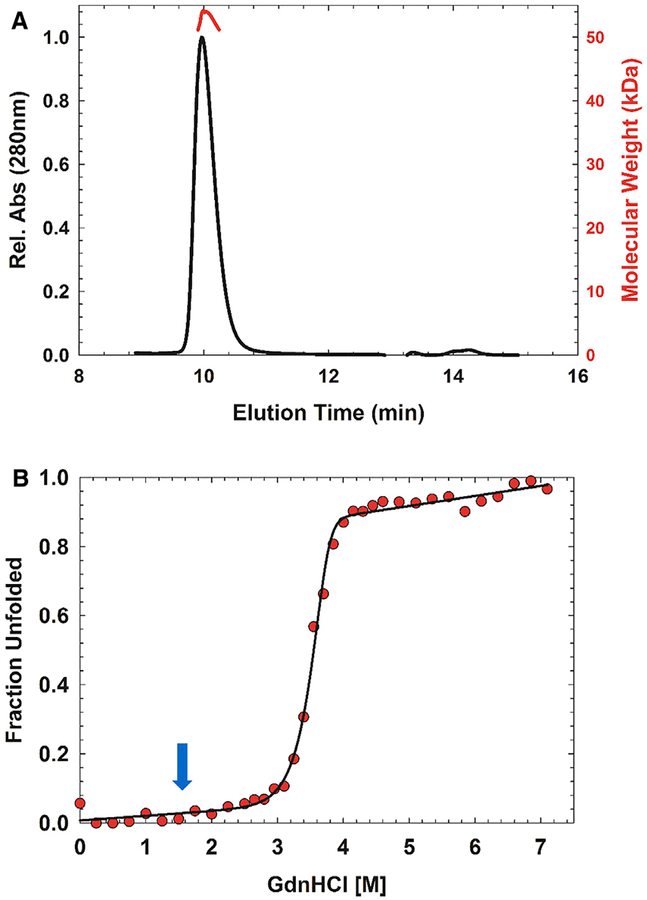
(A) P74–26 gp87 forms a stable trimer in solution as determined by size-exclusion chromatography-multi-angle light scattering. Predicted molecular mass, 49 kDa; measured molecular mass, 52 kDa.
(B) Representative equilibrium fraction unfolding curve of P74–26 gp87 at 5 μM shows a steep unfolding transition from 3 to 4 M GdnHCl; excitation, 295 nm; emission, 325 nm. The solid line represents the global fit to a model of trimer to three unfolded monomers. Blue arrow indicates the comparable transition midpoint of λ gpD unfolding.