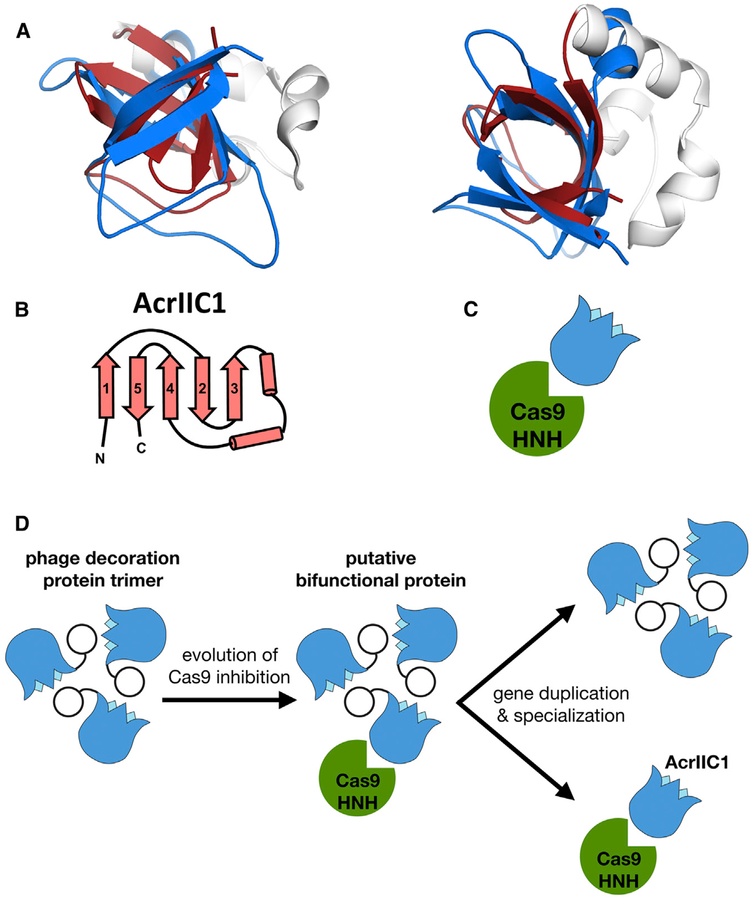
Figure 5. β tulip Domain Suggests Evolution of Acr Proteins from Phage Structural Proteins.
(A) Structural alignment of P74–26 gp87 β tulip domain (blue) with the anti-CRISPR protein AcrIIC1 (β tulip in red; PDB: 5VGB).
(B) Topology diagram of AcrIIC1 reveals conserved β tulip domain architecture.
(C) Cas9 binds stem side of the AcrIIC1 β tulip domain rather than the bloom side.
(D) Decoration protein trimers form interactions with neighboring subunits through the “bloom” end of the β tulip domain, leaving the “stem” end exposed. Anti-CRISPR AcrIIC1 binds to the Cas9 HNH domain through the stem end of the β tulip domain and may have evolved from a bifunctional phage decoration protein.