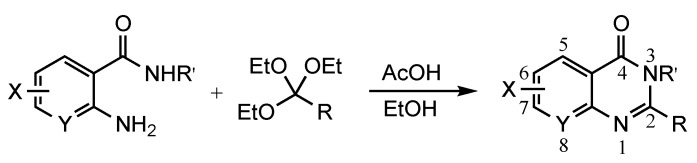
Table 1.
Synthesis of quinazolin-4(3H)-ones.
| Substrate | Product | R | Method a | Yield (%) |
|---|---|---|---|---|
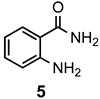
|
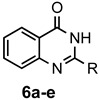
|
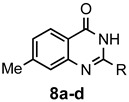
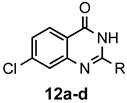
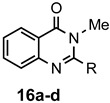
a: Me b: Et c: Pr d: P he: H |
1 1 1 1 1 |
92 89 93 81 87 |
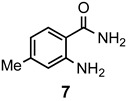
|
|
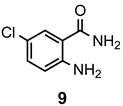
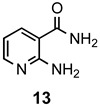
a: Me b: Et c: Pr d: Ph |
2 2 2 2 |
90 91 ND b 84 |
|
|
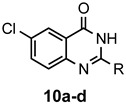
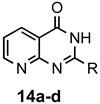
a: Me b: Et c: Pr d: Ph |
2 2 2 1(2) |
92 92 88 33(97) |
|
|
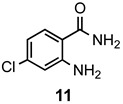
a: Me b: Et c: Pr d: Ph |
2 2 2 1(2) |
94 93 89 7(98) |
|
|
a: Me b: Et c: Pr d: Ph |
1(2) 1(2) 1(2) 1(2) |
71(86) 70(92) 55(87) 68(80) |
|
|
a: Me b: Et c: Pr d: Ph |
2 2 2 2 |
87 83 ND b 89 |
|
|
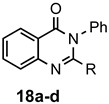
a: Me b: Et c: Pr d: Ph |
2 2 2 2 |
95 92 ND b 95 |
|
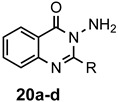
|
a: Me b: Et c: Pr d: Ph |
1(2) 2 2 2 |
21(82) 80 ND b 95 |
a For 1 equiv of the 2-aminobenzamide: Method 1: 1.5 equiv of orthoester, 2 equiv of AcOH, 3 mL of EtOH, 78 °C, 12–24 h; Method 2: 2–3 equiv of orthoester, 3 equiv of AcOH, 3 mL of EtOH, 110 °C, pressure tube, 12–72 h; b ND = not done; c Prepared from 2-nitrobenzoic acid as described in the experimental section.