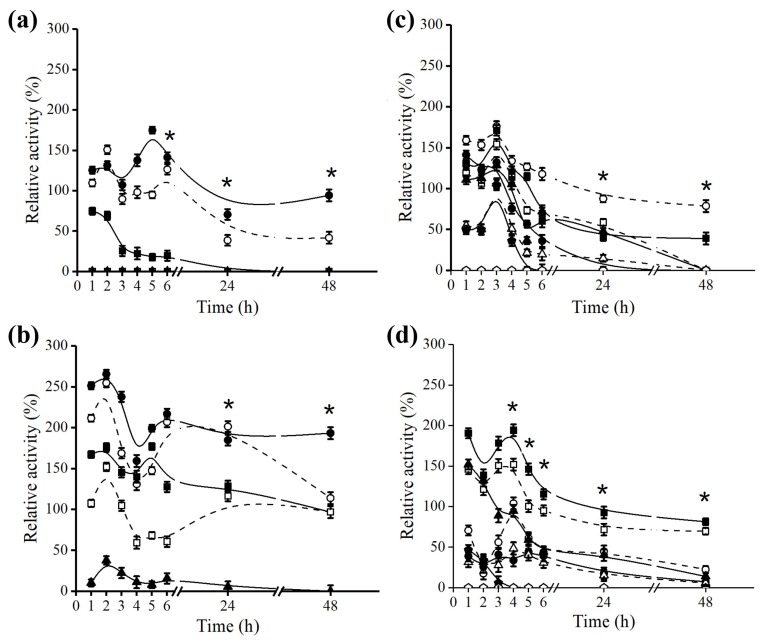
Figure 4.
Thermal and pH stabilities of F. oxysporum lipase: Thermal stability of crude extract (a) and derivative (b). pH stabilities of crude extract (c) and derivative (d). Thermal inactivation was performed in McIlvaine buffer pH 7.0 at the following temperatures; (●) 30 °C; (○) 40 °C; (■) 50 °C; (□) 60 °C and (▲) 70 °C. The pH stability was performed at 40 °C with McIlvaine buffer (pH 3–8) and 200 mM glycine buffer (pH 9 and 10); (●) pH 3.0; (○) pH 4.0; (■) pH 5.0; (□) pH 6.0; (▲) pH 7.0; (△) pH 8.0; (♦) pH 9.0 and (◊) pH 10.0. Lipase activity was obtained by 0.003% pNPP assay and relative activity was expressed according to the initial activity of the derivative prior to incubation at different pH and temperatures at several time intervals. The 100% of relative activity was considered as 440 U of F. oxysporum lipase activity immobilized on Octyl measured at the beginning of experiment. Other specifications are described in Section 3.8 and Section 3.9. The symbol (⋆) above an icon indicates the difference in significance between pH/temperatures for the same period of interest. The statistics used was One-Way ANOVA with ⋆ p < 0.05. The values were expressed in mean ± SEM, representing n = 3 for each group. SEM is less than 1% of mean value for the experiment set (error bars are not evident, as they lie within the area of the symbol). The statistics to evidence the difference between immobilized and crude extract are described in the text.