Table 2.
Optimization of the acetylenylation conditions for N,O-acetal TMS ethers.
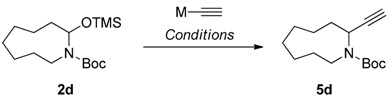
| Entry a | M | Additive | Lewis acid | Solvent | Yield b |
|---|---|---|---|---|---|
| 1 | MgBr | CuBr·SMe2 | BF3·OEt2 | THF | ND c |
| 2 | MgBr | BF3·OEt2 | THF | ND c | |
| 3 | Li | BF3·OEt2 | THF | ND c | |
| 4 | SiMe3 | BF3·OEt2 | CH2Cl2 | ND c | |
| 5 | n-Bu3Sn | BF3·OEt2 | CH2Cl2 | 58 | |
| 6 | n-Bu3Sn | BF3·OEt2 | THF | ND c | |
| 7 | n-Bu3Sn | BF3·OEt2 | PhCH3 | 37 | |
| 8 | n-Bu3Sn | BF3·OEt2 | Et2O | 29 | |
| 9 | n-Bu3Sn | SnCl4 | CH2Cl2 | ND c | |
| 10 | n-Bu3Sn | TiCl4 | CH2Cl2 | ND c | |
| 11 | n-Bu3Sn | TMSOTf | CH2Cl2 | 46 |
a All reactions were performed using 0.2 mmol of 2d in dry solvent (2 mL) by adding the additive (if any) at −50 °C. The nucleophile was then added at the same temperature followed by the addition of BF3·OEt2 at −78 °C and stirring for 1 h. The reaction mixture was slowly warmed to r.t. over 2 h before quenching with Et3N. b Isolated yields. c Not detected.
