Table 3.
Acetylenylation of N,O-acetal TMS ethers.
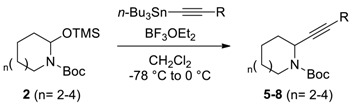
| Entry a | n | R | Yield b |
|---|---|---|---|
| 1 | 1 | H | 30 |
| 2 | 1 | SiMe3 | 29 |
| 3 | 1 | Me | 35 |
| 4 | 1 | Ph | 31 |
| 5 | 2 | H | 28 |
| 6 | 2 | SiMe3 | 38 |
| 7 | 2 | Me | 89 |
| 8 | 2 | Ph | 88 |
| 9 | 3 | H | 84 |
| 10 | 3 | SiMe3 | 36 |
| 11 | 3 | Me | 82 |
| 12 | 3 | Ph | 68 |
| 13 | 4 | H | 58 |
| 14 | 4 | SiMe3 | 64 |
| 15 | 4 | Me | 80 |
| 16 | 4 | Ph | 81 |
a All reactions were performed using 0.2 mmol of 2a–d in dry solvent (2 mL) by first adding the nucleophile at −78 °C followed by adding BF3·OEt2 and stirring for 1 h. The mixture was then slowly warmed to r.t. over 1 h before quenching with Et3N. b Isolated yields.
