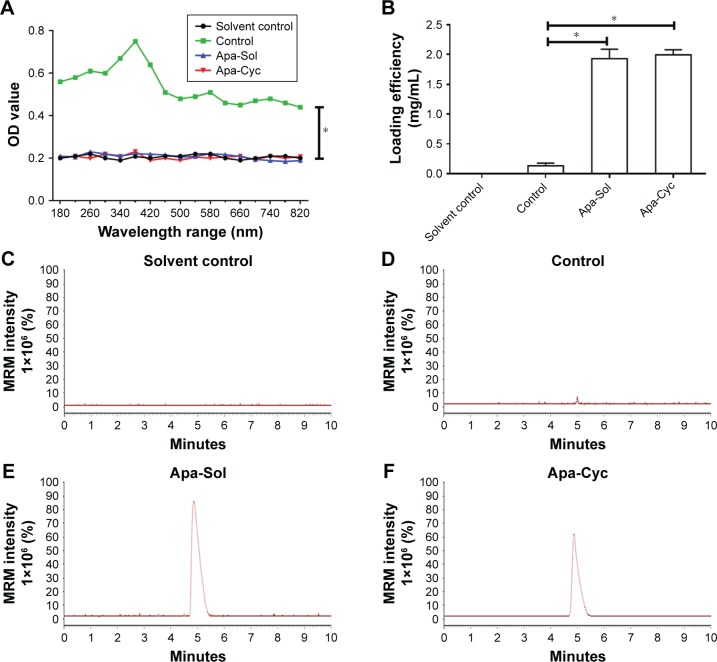
Figure 1.
Preparation of apatinib formulations.
Notes: Apatinib was 1) simply mixed with ddH2O to form an apatinib suspension (named Control); 2) dissolved in organic solvents and diluted in ddH2O to form an apatinib solution (named Apa-Sol); and 3) mixed with cyclodextrin to form an apatinib–cyclodextrin inclusion complex (named Apa-Cyc). (A) Apatinib formulations were scanned with a microplate reader to obtain the OD values of apatinib formulations (Control, Apa-Sol, and Apa-Cyc) measured under a series of wavelengths. Cyclodextrin solution in ddH2O was used as a solvent control. (B) Apatinib suspension was filtered with a 0.22 µm pore diameter filter membrane. Apatinib formulations (Control, Apa-Sol, and Apa-Cyc) were extracted by ACN for LC-MS/MS examination. (C–F) The typical photographs of Solvent Control (C), Control (apatinib suspension) (D), Apa-Sol (E), and Apa-Cyc (F) by LC-MS/MS. *P<0.05.
Abbreviations: ACN, acetonitrile; Apa-Cyc, apatinib–cyclodextrin inclusion complex; Apa-Sol, apatinib solution; ddH2O, double-distilled H2O; LC-MS/MS, liquid chromatography mass spectrometry/mass spectrometry; MRM, multi reaction monitoring.