Fig. 4. The humoral immune response to rNT-DsrAI is similar when administered with Freund’s, MPL, CpG or imiquimod adjuvant, and improved with intramuscular compared to subcutaneous immunization route.
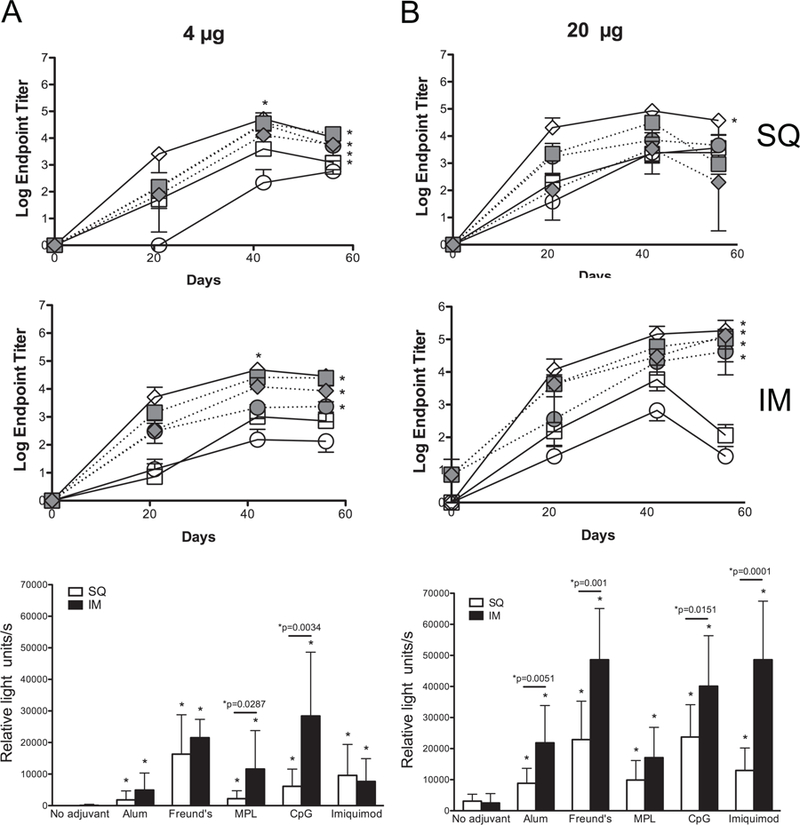
Groups of 5 mice were immunized either with 4 (A) or 20 (B) µg of rNT-DsrAI in the absence or presence of 5 different adjuvants (Alum, Freund’s, MPL, CpG and Imiquimod) following subcutaneous (SQ, top) or intramuscular (IM, middle) routes. Mice were immunized and bled three times, three weeks apart (days 0, 21, and 42), and bled at day 56. Top and middle, means ± standard deviations of endpoint titers of individual antisera after SQ or IM immunization, respectively. Bottom, reactivity of individual antisera from day 56 to viable, homologous H. ducreyi strain 35000HP (white column, SQ route; black column, IM route). * indicates p<0.05 as compared to the response obtained with “no adjuvant” at that specific dose/route for the entire time of the trial (56 days); for whole cell binding ELISAs (bottom), “*p=“ compares SQ and IM routes for pairs of doses and adjuvants using an unpaired t-test.
