Figure 3. Mechanism-based trapping of PDI substrates.
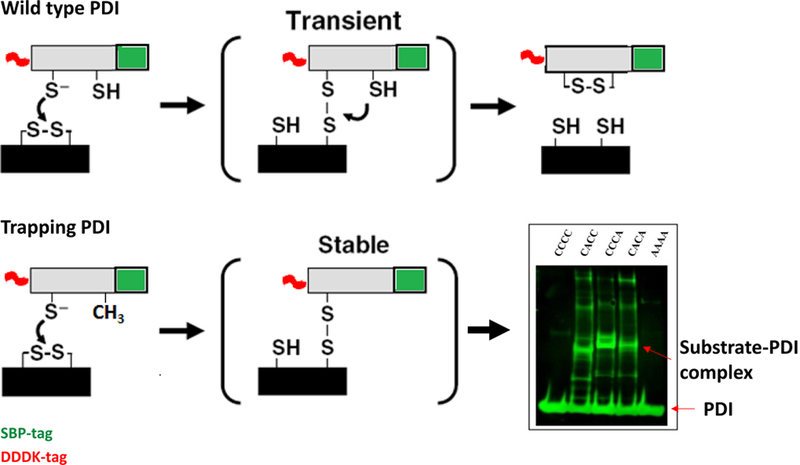
Reduction of a substrate disulfide by PDI occurs through transient formation of a mixed disulfide between the N-terminal Cys in the PDI CGHC motif and a Cys in the substrate, as depicted in the top panel. Resolution of the mixed disulfide requires the C-terminal Cys of the CGHC motif. Mutation of the C-terminal Cys to alanine (Ala) in the PDI active site (CGHC to CGHA) makes this mixed PDI-substrate disulfide stable, as depicted in the lower panel. These PDI-substrate complexes can then be isolated and analyzed. The Western blot on the right shows one such reaction where no substrate is isolated with the use of wild-type PDI (CCCC) or inactive PDI (AAAA), but only with PDI trapping mutants with C-terminal Cys modified to Ala in one (CACC and CCCA) or both (CACA) catalytically active domains.
