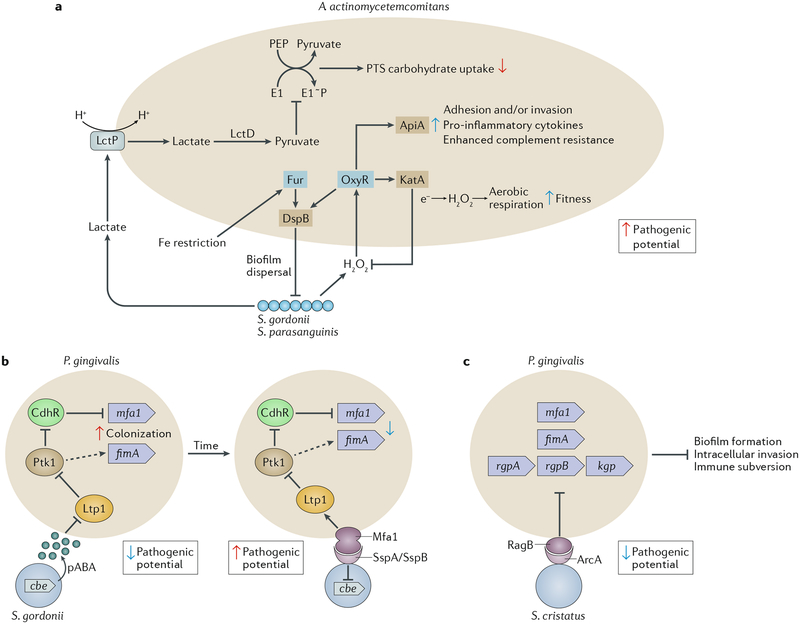
Fig. 2 |. Interactions among bacterial species that affect nososymbiocity.
Oral bacteria interact through multiple pathways that can be separated both spatially and temporally. a | Streptococcus gordonii and Streptococcus parasanguinis produce hydrogen peroxide, to which Aggregatibacter actin-omycetemcomitans responds by activation of the OxyR transcriptional regulator; consequently, transcription of both apiA and katA is elevated. Higher levels of the ApiA surface protein increases complement resistance and will also potentially induce intracellular invasion and pro-inflammatory cytokine production. KatA (a catalase) degrades the hydrogen peroxide produced by both streptococci and neutrophils, thus protecting A. actino-mycetemcomitans from oxidative damage64,66,154–156. OxyR also regulates production of dispersin B (DspB), an enzyme that degrades the biofilm matrix and facilitates dispersal of A. actinomycetemcomitans65. Hydrogen peroxide increases the bioavailability of oxygen, and, in response, A. actinomycetemcomitans shifts from a primarily fermentative to a respiratory metabolism, an interaction referred to as cross respiration157. Respiratory metabolism enhances the growth and fitness of A. actinomycetemcomitans in vivo. Transport of streptococcal lactate into A. actinomycetemcomitans through the proton-driven lactate permease (LctP) leads to conversion to pyruvate by lactate dehydrogenase (LctD). Pyruvate suppresses autophosphorylation of E1, which then decreases uptake of phosphotransferase system (PTS) carbohydrates such as glucose155. Preferential utilization of lactate through this carbon resource partitioning gives a competitive advantage to A. actinomycetemcomitans in the presence of organisms that can metabolize glucose more efficiently. Communities of A. actinomycetemcomitans and S. gordonii also become restricted for iron. The Fur transcriptional regulator of A. actinomycetemcomitans responds to iron limitation and induces upregulation of the gene encoding DspB, which will release A. actinomycetemcomitans from biofilms158. A. actinomycetemcomitans responses to both oxidative stress and iron restriction thus involve DspB activity and re-localization, and in vivo A. actinomycetemcomitans maintains an optimal distance from streptococci in communities that are synergistically virulent65. b | Interactions between Porphyromonas gingivalis and S. gordonii resulting from metabolite (4-amino benzoate (pABA)) perception (left) and direct contact (right). pABA secreted by S. gordonii inactivates the P. gingivalis tyrosine phosphatase Ltp1. Dephosphorylation and inactivation of the tyrosine kinase Ptk1 is thus reduced. Ptk1 phosphorylates and inactivates the transcription factor CdhR, which is a repressor of the mfa1 gene. Ptk1 activity also converges on expression of the fimA gene. Expression of both fimbrial adhesins is increased, and in this mode P. gingivalis is primed for attachment to S. gordonii. However, nososymbiocity is reduced, and pABA-treated P. gingivalis are less pathogenic in animal models. Engagement of Mfa1 with the streptococcal SspA or SspB surface protein increases Ltp1 and reverses information flow through the Ltp1-Ptk1 axis. In addition, Mfa1-SspA/SspB binding suppresses expression of chorismate binding enzyme (Cbe), which is responsible for pABA production. Prolonged physical interaction between P. gingivalis and S. gordonii leads to increased nososymbiocity, and dual infection of animal models causes more alveolar bone loss than P. gingivalis infection alone60–63,159,160. c | Streptococcus cristatus arginine deiminase (ArcA) interacts with the P. gingivalis surface protein RagB. Signal transduction results in downregulation of genes encoding the FimA and Mfa1 component fimbriae along with the arginine-specific (RgpA or RgpB) and lysine-specific (Kgp) gingipain proteinases. Adhesion, biofilm formation, epithelial cell invasion and degradation of cytokines are consequently reduced and nososymbiocity is suppressed72–74. Part a adapted with permission from REF56, Wiley-VCH.