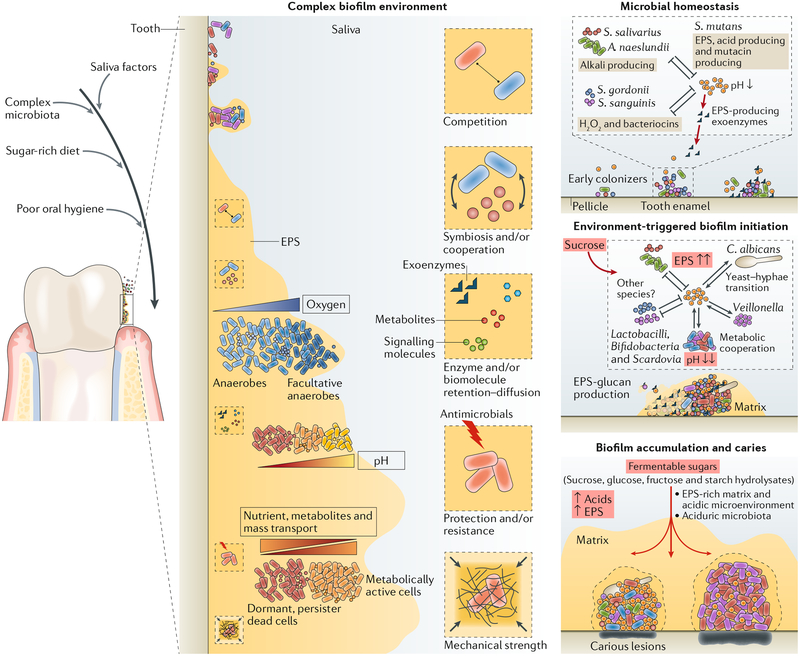
Fig. 3 |. Diet-microbiota interactions trigger the assembly of cariogenic biofilm microenvironment.
In the oral microbial community on tooth surfaces, social interactions begin with primary colonizers that can rapidly attach and then co-adhere with later colonizers. Microorganisms can interact physically and metabolically to determine the initial biofilm community. Both antagonistic and cooperative interactions can occur, and these dynamically change according to the host diet, and other factors such as salivary dysfunction, fluoride exposure and oral hygiene. In particular, dietary sucrose provides a substrate for extracellular polysaccharide production and synthesis of organic acids by acidogenic microorganisms. The extracellular matrix, which also contains other biomolecules (extracellular DNA (eDNA) and bacterial or host-derived proteins), provides a multi-functional scaffold for spatial organization, mechanical coherence and interbacterial interactions. The matrix can trap or sequester substances, which, in combination with diffusion-modifying properties, can generate a variety of chemical and protective microenvironments. Biofilms thus become persistently adhered to the surface and recalcitrant to antimicrobial action. S. mutans has a key pathogenic role as an EPS-matrix producer, acidogenic and aciduric organism. With frequent dietary sugar exposure, continued bacterial metabolism of carbohydrates and reduced accessibility to salivary buffering systems causes the microenvironment within the matrix to become increasingly and constantly acidic. As the biofilm accumulates, the microenvironment also becomes progressively anaerobic (hypoxic). In a feedforward loop, microbial diversity decreases as an aciduric microbiota predominates. If the biofilm is not removed, persistent low-pH conditions at the tooth-biofilm interface shift the demineralization-remineralization balance towards net mineral loss from the tooth enamel, leading to the development of a carious lesion. EPS, extracellular polymeric substance. Adapted with permission from REF7, Cell Press.