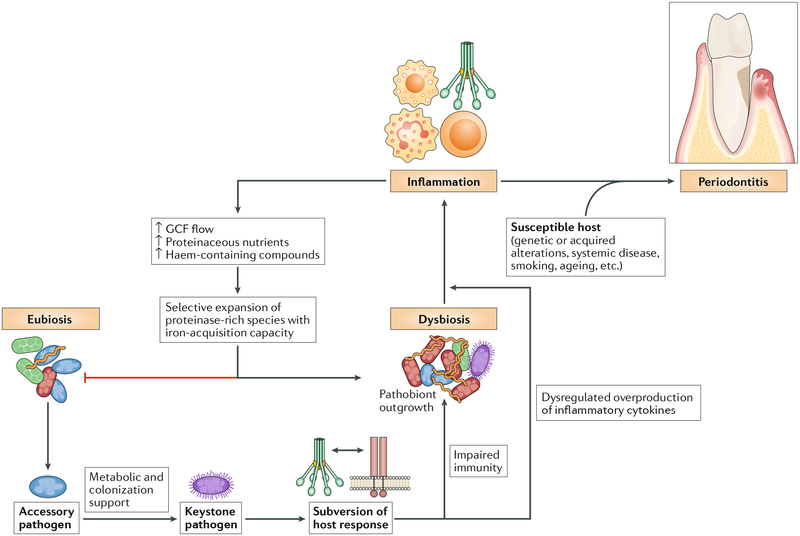
Fig. 4 |. Reciprocally reinforced interactions between dysbiosis and inflammation drive chronic periodontitis.
Colonization by keystone pathogens (for example, Porphyromonas gingivalis) aided by accessory pathogens (for example, Streptococcus gordonii) leads to impaired innate host defence and promotion of inflammation (for example, by subverting complement-Toll-like receptor (TLR) crosstalk in neutrophils and other myeloid cells)60,102,117,119. These alterations contribute to the emergence of dysbiosis (quantitative and compositional changes in the periodontal microbiota). Inflammation worsens dysbiosis by increasing the flow of gingival crevicular fluid (GCF), which, as a result of inflammatory tissue destruction, carries degraded collagen and haem-containing compounds into the gingival crevice, where dysbiotic communities develop. These molecules are selectively used by proteolytic and asaccharolytic bacteria with iron-acquisition capacity. By contrast, health-associated (eubiotic) species cannot capitalize on the new environmental conditions and are outcompeted. This imbalance drives dysbiosis, which further exacerbates inflammation, culminating in periodontitis in susceptible individuals. The ability of inflammation and dysbiosis to positively reinforce each other in a self-sustained feedforward loop may contribute to the chronicity of periodontitis.