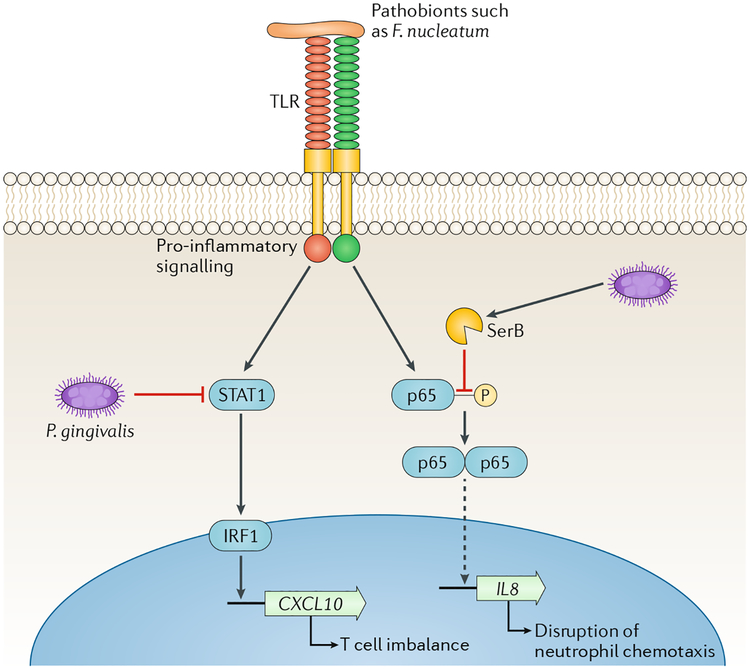
Fig. 6 |. Localized chemokine paralysis.
Oral pathobionts such as Fusobacterium nucleatum are recognized by Toll-like receptors (TLRs) on epithelial cell surfaces, which leads to the activation of pro-inflammatory signalling pathways. The keystone pathogen Porphyromonas gingivalis can manipulate these pathways and cause a targeted and precise reduction in the production of specific chemokines. Inactivation of STAT1 by P gingivalis leads to reduced expression of CXCL10, which is controlled by the IRF1 transcription factor124. Intracellularly, P. gingivalis secretes SerB, a serine phosphatase that specifically dephosphorylates the serine 536 residue of the p65 NF-κB subunit, thus inhibiting formation and nuclear translocation of NF-κB-p65 homodimers. Transcription of the IL8 gene is reduced and the IL-8 neutrophil gradient is disrupted125. These chemokine paralysis activities will be localized to tissue adjacent to, or containing, P. gingivalis, and in animal models supersede the effects of community pathobionts163. The continuous recalibration of host cell signalling pathways also limits the temporal extent of the phenomenon, which may contribute to the cyclical nature of periodontal tissue destruction. Adapted with permission from REF10, Cell Press.