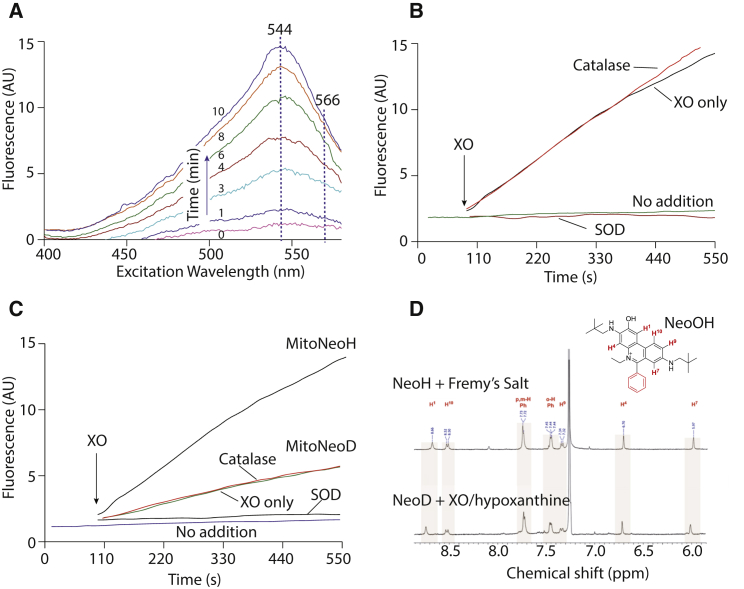
Figure 3.
Fluorescence and NMR Analysis of Reaction of MitoNeoH/D with O2⋅−
(A) Excitation fluorescence spectra over time. MitoNeoH (10 μM) was incubated at 37°C in KCl buffer with 1 mM hypoxanthine (HX) and 5 mU/mL xanthine oxidase (XO) and the excitation spectrum was assessed at various times using an emission wavelength of 605 nm.
(B and C). Time courses of reaction of MitoNeoH (B) or MitoNeoD (C) with O2⋅−.
MitoNeoH or MitoNeoD (10 μM) was incubated with 1 mM HX and 5 mU/mL XO, in the presence of 10 μg/mL SOD or 50 U/mL catalase in KCl buffer at 37°C. Excitation and emission wavelengths were 544 and 605 nm, respectively.
(D) 1H NMR analysis of reaction product of NeoD with O2⋅−. The upper 1H NMR spectrum is of NeoOH in CDCl3, synthesized from NeoH using Fremy's salt. For the lower spectrum, NeoD (100 μM) was exposed to O2⋅− by incubation with XO (0.5 U/mL) and HX (1 mM) for 3 hr in a 1:0.5:3.5 mixture of EtOH:PBS:H2O and then extracted into CHCl3, purified by HPLC and the 1H NMR spectrum obtained. The expansion is of the aromatic region of the spectrum where only the numbered protons of the phenanthridinium moiety and those on the 6-phenyl group appear (red on the MitoNeoOH structure).
See also Figure S4.