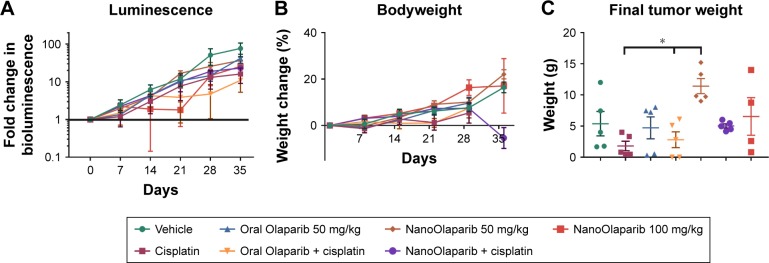
Figure 5.
NanoOlaparib administered twice weekly at 50 or 100 mg/kg as a monotherapy or in combination with cisplatin in a Brca2−/−, Tp53−/−, Pten−/− intraperitoneal spread model showed no efficacy after 5 weeks.
Notes: (A) Bioluminescence measurements show the disease progression over 5 weeks of treatment. (B) Bodyweight measurements indicate no apparent gross toxicity in any of the treatment groups. (C) Final tumor weights serve as a quantitative measure of disease burden at the end of the study. The data did not follow a normal distribution therefore significance was tested with Kruskal–Wallis one-way ANOVA followed by Dunn’s test at α=0.05. Statistically significant decreases in tumor weights were seen in both cisplatin-treated tumors vs NanoOlaparib and oral Olaparib + cisplatin compared to NanoOlaparib, respectively (*P<0.05).