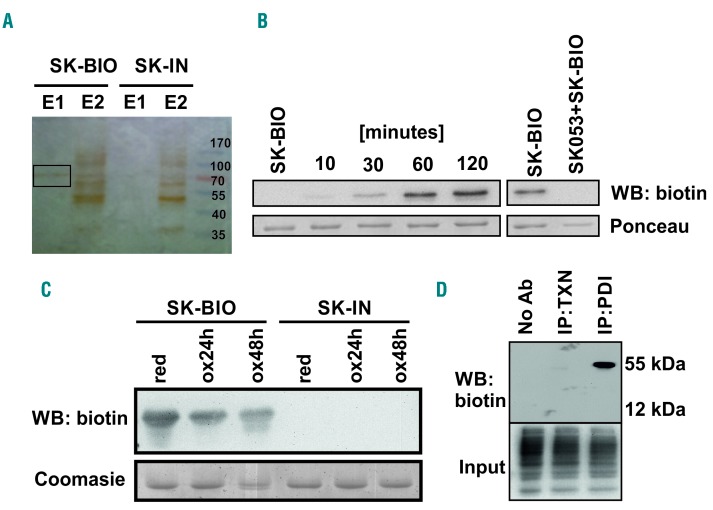
Figure 2.
SK053 binds to protein disulfide isomerase. (A) HL-60 cells were incubated with 100 μM SK-BIO and lysed. Proteins binding SK-BIO were precipitated with avidin-coated beads and eluted with buffers E1 and E2 as described in the Methods section. Next, the samples were separated by SDS-PAGE. A band obtained with buffer E1 (black rectangle) was excised from silver-stained gel and analyzed by mass spectrometry. An inactive biotinylated SK053 analog (SK-IN) was used as a negative control. A representative result of a series of experiments is presented. (B) Recombinant human (rhu) PDI was incubated with a 10× molar excess of SK-BIO for 10–120 min at 37°C (left) or preincubated for 1 h with a 10× molar excess of SK053 followed by 1 h incubation with a 10× molar excess of SK-BIO (right). Next, proteins were separated by SDS-PAGE followed by western blotting (WB) using anti-biotin monoclonal antibody. Membranes stained with Ponceau red served as loading controls. A representative result of a series of experiments is presented. (C) Reduced (red) and oxidized (ox) rhuPDI were incubated with SK-BIO followed by immunoblotting for biotin. Gels stained with Coomasie blue served as loading controls. SK-IN was used as a negative control. A representative result of a series of experiments is presented. (D) The lysates of HL-60 cells were incubated with SK-BIO followed by biotin immunoprecipitation and western blotting. Biotin detection in total cell lysates (input) was used as a loading control. Lysate incubated with protein G agarose beads in the absence of antibodies was used as a negative control (No Ab). A representative result of a series of experiments is presented. IP: immunoprecipitation; TXN: thioredoxin.