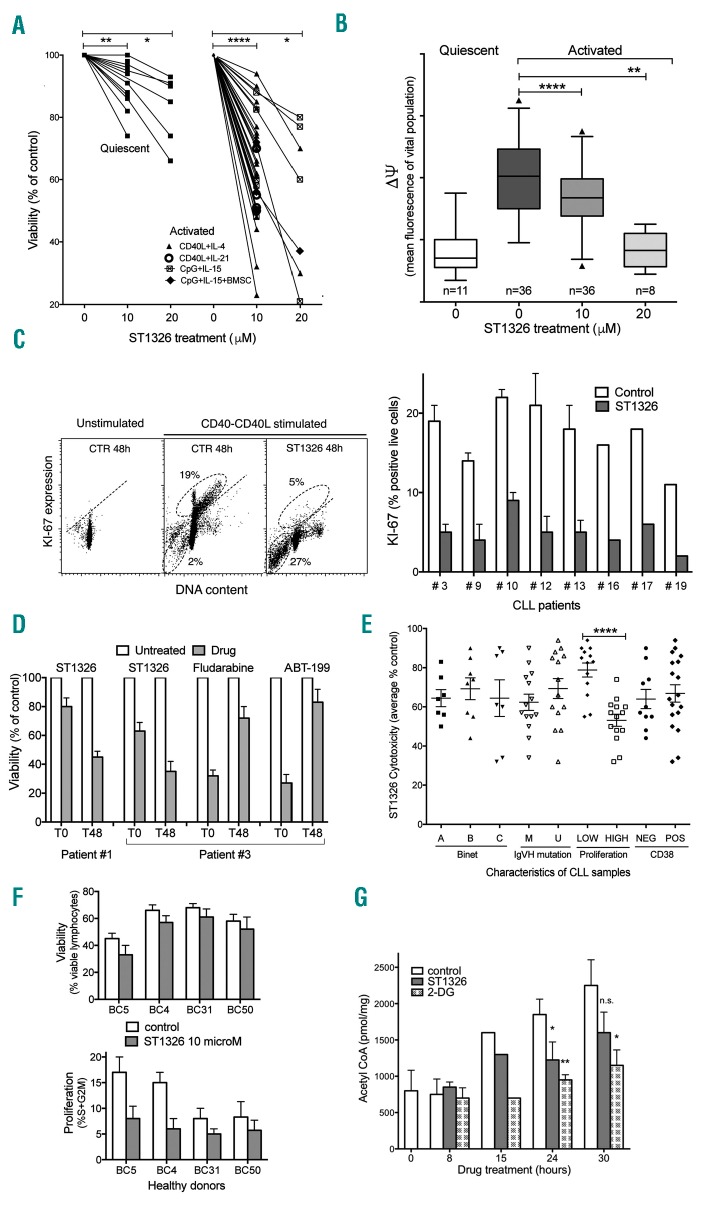
Figure 1.
ST1326 kills proliferating chronic lymphocytic leukemia cells. (A) Cell viability assessed by flow cytometric propidium iodide exclusion tests on quiescent or CD40L-stimulated cultures of leukemic cells from 26 CLL patients untreated or treated with ST1326 (48 h). CLL cells were obtained from peripheral blood of CLL patients, after informed consent according to the Declaration of Helsinki. Cell activation was achieved either through CD40L-NIH-3T3 murine fibroblasts + IL-4 (number of independent experiments, n=22) or IL-21 (n=4), or by CpG/ODN2006 (hTLR9 ligand) +IL-15, in the absence (n=7) or presence (n=3) of bone marrow stromal cells (BMSC). Mean (range) values of the reduction of viability (% control) induced by 10 μM ST1326 were: 64.8 (23–94), 56.5 (50–70), 68 (48–88) and 61 (55–72) for the four different activation systems, respectively. The statistical significance of differences was evaluated by a two-sided Wilcoxon signed rank test. *P≤0.05; **P≤0.01; ***P≤0.001; ****P≤0.0001. (B) Mitochondrial transmembrane potential (ΔΨ) gated on the vital cell population, as measured by DiOC6 uptake and expressed as flow cytometric fluorescence intensity of the propidium iodide-excluding cell population (with intact plasma membrane), on quiescent or CD40L-stimulated cultures of leukemic cells from 26 CLL patients untreated or treated with ST1326 from the beginning of stimulation (48 h). The number of samples for each condition is indicated. The statistical significance of differences was analyzed by a t-test two-sided Wilcoxon signed rank test. *P≤0.05; **P≤0.01; ***P≤0.001; ****P≤0.0001. (C) Left: flow cytometric distributions of DNA content/KI67 expression for one representative CLL sample. The CD40L-stimulated cells were tested in the absence or presence of ST1326. Gates identify KI67+ cells (above the negative-threshold dotted line) and sub-G1 events (apoptotic cells and bodies, below). Right: %KI-67+ cells from CLL samples of eight CLL patients, tested with or without addition of ST1326. (D) Cell viability in cells from two CLL patients exposed to a 40 h drug ‘pulse’, either concomitantly with CD40L-stimulation (T0) or after 48 h (T48). The different ST1326 sensitivities of CLL cells at T0 and T48 is evident. In contrast, the effect of fludarabine (10 μM) and ABT-199 (5 nM) was remarkably impaired if the cells were exposed to the drugs at T48. (E) Effect of ST1326 on average cell viability of CD40L-stimulated CLL cells. The patients were grouped according to disease stage (Binet A, B or C), immunoglobulin gene mutational status [unmutated (U-CLL), <2% mutations in IGVH genes); mutated (M-CLL), ≥2% mutations in IGVH genes], proliferative response to CD40L-stimulation (‘low’ or ‘high’ if % cells in the S+G2M cell cycle phase after 72 h stimulation was ≤ or > 8%, respectively), and CD38 expression (cut-off: 30%). The statistical significance of differences was assessed by a two-sided Mann-Whitney test. Where no indication is reported, the difference was not statistically significant. *P≤0.05; **P≤0.01; ***P≤0.001; ****P≤0.0001. (F) Normal B cells from healthy volunteers were purified by magnetic beads (negative-selection) and activated by co-culturing with CD40L+IL-4 and treated for 48 h with 10 μM ST1326. Cell death and proliferation were measured by flow cytometry. Viability was unaffected, while cell proliferation (% cells in S+G2M phase) was reduced in two samples. (G) Acetyl CoA levels in leukemic cells from one CLL patient stimulated for 24 h and then exposed to ST1326 (10 μM) or 2-DG (5 mM). Data obtained by colorimetric tests using the PicoProbe Acetyl CoA Assay Kit from AbCam (http://www.abcam.com/), are reported as mean ± SD of three experiments (two settings with CpG-and one with CD40L-stimulus). The levels of intracellular acetyl CoA increase during CLL cell activation, and ST1326 affects the increase rate. 2-DG was used as a control. *P≤0.05; **P≤0.01; ***P≤0.001; ****P≤0.0001.