Abstract
The evaluation of patients presenting to the emergency department with suspected acute coronary syndrome (ACS) remains a clinical challenge. The traditional assessment includes clinical risk assessment based on cardiovascular risk factors with serial electrocardiograms and cardiac troponin measurements, often followed by advanced cardiac testing as inpatient or outpatient (i.e. stress testing, imaging). Despite this costly and lengthy work-up, there is a non-negligible rate of missed ACS with an increased risk of death. There is a clinical need for diagnostic strategies that will lead to rapid and reliable triage of patients with suspected ACS. We provide an overview of the evidence for the role of highly sensitive troponin (hsTn) in the rapid and efficient evaluation of suspected ACS. Results of recent research studies have led to the introduction of hsTn with rapid rule-in and rule-out protocols into the guidelines. Highly sensitive troponin increases the sensitivity for the detection of myocardial infarction and decreases time to diagnosis; however, it may decrease the specificity, especially when used as a dichotomous variable, rather than continuous variable as recommended by guidelines; this may increase clinician uncertainty. We summarize the evidence for the use of coronary computed tomography angiography (CTA) as the rapid diagnostic tool in this population when used with conventional troponin assays. Coronary CTA significantly decreases time to diagnosis and discharge in patients with suspected ACS, while being safe. However, it may lead to increase in invasive procedures and includes radiation exposure. Finally, we outline the opportunities for the combined use of hsTn and coronary CTA that may result in increased efficiency, decreased need for imaging, lower cost, and decreased radiation dose.
Keywords: Acute coronary syndrome, Acute chest pain, Highly sensitive troponin, Coronary computed tomography angiography
Current standards in the evaluation of patients with suspected acute coronary syndrome in the emergency department
The evaluation of patients presenting to the emergency department (ED) with suspected acute coronary syndrome (ACS) remains a clinical challenge. Acute chest pain is one of the most common chief complaints in the ED in Europe and the USA: among 100 million ED visits every year, ∼6 million patients present with acute chest pain.1,2 Ultimately, a cardiac cause is suspected in ∼4 million patients. However, only approximately one-third of patients are eventually diagnosed with an ACS. On the other hand, among patients who are diagnosed with non-cardiac chest pain, 1–4% suffer ACS, and these missed diagnoses are associated with substantially higher risk for death.3,4
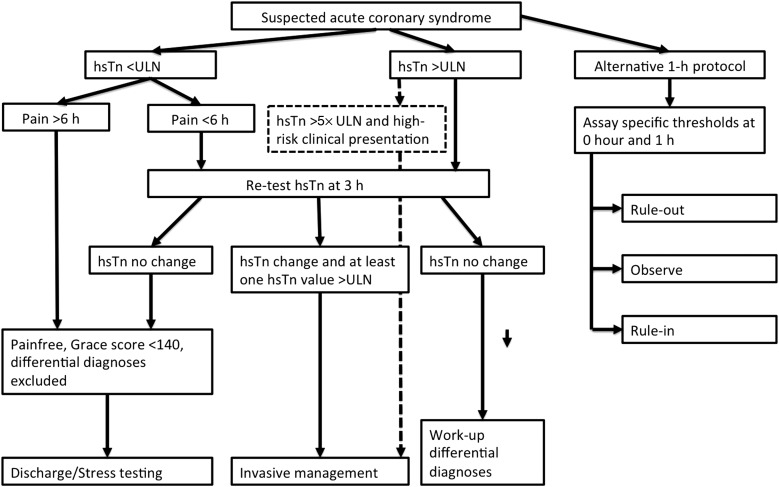
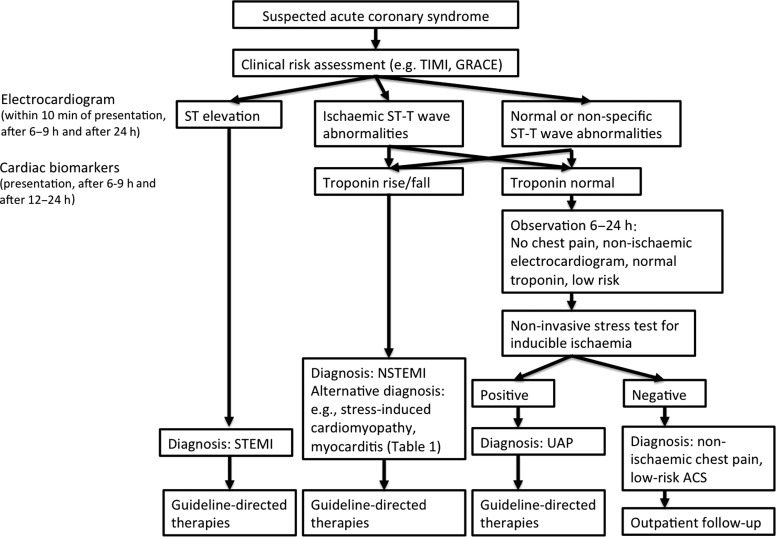
Practice guidelines of the European Society of Cardiology and American Heart Association/American College of Cardiology provide algorithms for the evaluation of suspected ACS.5,6 The algorithms acknowledge the limitations of chest pain history and clinical risk factors. While these factors are important in the evaluation, they do not allow for a definitive exclusion of ACS.4–6 The standard of care for subjects with suspected ACS includes electrocardiograms and cardiac troponin (Tn) measurements (Figures 1 and 2). Differences exist between guidelines and practice in the USA and Europe due to the lack of US Food and Drug Administration approval for highly sensitive Tn (hsTn) assays in the USA. In Europe, clinicians have had now >5 years of experience with hsTn and the most recent guidelines include hsTn with baseline and 3-h assessment in the recommended algorithm (Figure 1).5 Furthermore, a possibility of rapid 1-h protocols is suggested for centres with appropriate expertise and availability of assays provided in the guidelines.5 In contrast, longer work-up with serial measurements of conventional Tn at least at 6 h is currently recommended in the USA (Figure 2).5,6
Figure 1.
Simplified algorithm for the management of patients with suspected acute coronary syndrome based on European Society of Cardiology guidelines.5
Figure 2.
Simplified algorithm for the management of patients with suspected acute coronary syndrome based on American Heart Association/American College of Cardiology guidelines.6
While the hsTn methods have improved sensitivity for the detection of myocardial necrosis compared with conventional assays, the specificity of hsTn assays for the clinical diagnosis of myocardial infarction (MI) is lower when evaluated as a dichotomous value. Such approach may lead to the need for repeat Tn testing, evaluation with advanced diagnostic testing with or without imaging, in-hospital observation, and high healthcare cost. European guidelines emphasize that hsTn should be interpreted as a quantitative marker with higher levels being associated with higher likelihood of MI.5 Whether using conventional Tn or hsTn, there will be a group of patients requiring further work-up and objective tests are needed to support clinical judgement. Given these challenges, there is a clinical need for diagnostic strategies that will lead to rapid and reliable triage of patients with suspected ACS. In this review, we discuss the potential roles of hsTn and coronary computed tomography angiography (CTA) as individual tests and also in combination for the evaluation of patients with suspected ACS in the ED.
Highly sensitive troponin assays
Conventional cardiac Tn assays have been used to detect acute MI for over two decades. For diagnosis, the third Universal Definition of MI Global Task Force recommended the use of a cardiac Tn cut-off that is the 99th percentile of a healthy patient population.7 The challenges of conventional Tn assays include higher limit of detection (typically 10–60 ng/L) and inaccuracy at very low concentrations of the biomarker (coefficient of variation at the 99th percentile value typically ≥10%) along with a time dependency to their detectability, given the need for substantial efflux of the cytosolic Tn pool into the blood stream.8,9 Conventional Tn methods require at least several hours from the onset of myocardial injury before becoming abnormal, therefore, leading to the ‘Tn blind interval’. In contrast, hsTn assays provide increased precision at or <99th percentile. Two criteria have to be met to define an assay as hsTn: (i) total imprecision (coefficient of variation) at the 99th percentile value ≤10% and (ii) measurable concentrations <99th percentile attainable at a concentration value above the assay's limit of detection for at least 50% (and ideally 95%) of healthy individuals.8,9 This increased sensitivity results in measurable values of hsTn in the majority of normal subjects and in subjects with prevalent cardiovascular disease. Therefore, the Study Group on Biomarkers in Cardiology of ESC emphasized that the elevation of hsTn alone is not sufficient to make a diagnosis of MI.9
Prognostic value of highly sensitive troponin in normal populations
Values >99th percentile provide prognostic information for future major cardiovascular events (MACE) in subjects free of clinical cardiovascular disease.10–12 In the population from Dallas Heart Study, hsTn >99th percentile when compared with below the limit of detection were associated with increased cardiac structural abnormalities [left ventricular (LV) hypertrophy and dysfunction] and with 2.8 times increased risk of all-cause mortality after adjustment.10 Similarly in Atherosclerosis Risk in Communities Study study, hsTn >99th percentile was associated with an increased risk of incidental coronary heart disease (hazard ratio 2.3), mortality (hazard ratio 4.0), and heart failure (hazard ratio 6.0).11 Finally, in the Framingham Heart Study, higher hsTn was associated with an increased risk of all-cause mortality, MACE, and heart failure (hazard ratio per standard deviation increase: 1.16, 1.28, and 1.18, respectively).12
Diagnostic performance of highly sensitive troponin for myocardial infarction
In the evaluation of patients with suspected MI, use of hsTn assays provides superior diagnostic accuracy with very high sensitivity and negative predictive value compared with conventional assays.13–18 The diagnostic accuracy is significantly improved particularly in patients who present within 3 h of symptom onset, thus resulting in excellent ability to exclude acute MI at an earlier time frame through rapid serial assessment (Figure 3).14,15,17,18 In addition to early identification of patients with MI, hsTn can re-classify up to one-third of patients with unstable angina pectoris to a new diagnosis of MI.19,20
Figure 3.
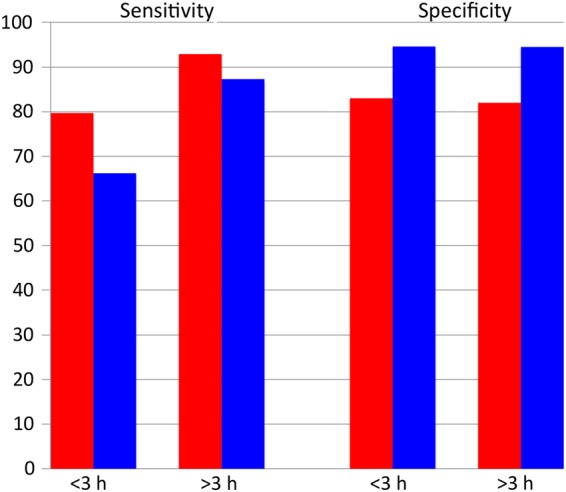
The diagnostic performance of highly sensitive troponin (red bars) when compared with conventional troponin (blue bars) for acute myocardial infarction in patients presenting <3 and >3 h of chest pain onset (adapted from Body et al.15). Highly sensitive troponin assay provides significantly improved sensitivity for the detection of myocardial infarction in patients presenting within 3 h of chest pain onset. There is no difference in the specificity in those who present <3 or >3 h of chest pain onset.
Importantly, however, increased sensitivity of the hsTn assays for the detection of small amounts of myocardial necrosis comes with the cost of modestly decreased specificity and misdiagnosis of MI, especially in patients with multiple complex comorbid conditions, or other diagnoses leading to myocardial necrosis (Table 2). While increasing prognostic value for adverse outcomes across a broad range of cardiovascular disease, loss of positive predictive value of an elevated hsTn for the clinical diagnosis of acute MI may therefore result in increase in downstream testing and costly interventions.20–23
Table 2.
Summary of randomized, controlled, and multicentre trials comparing coronary computed tomography angiography with standard of care in patients with suspected acute coronary syndrome in the emergency department
| Study | CT-STAT40 |
ACRIN46 |
ROMICAT II51 |
|||
|---|---|---|---|---|---|---|
| Population (n) | 699 | 1370 | 1000 | |||
| Mean age (years) | 50 | 49 | 54 | |||
| Women (%) | 54 | 53 | 47 | |||
| TIMI risk score | 0–4 | 0–2 | N/A | |||
| MI during index hospitalization (%) | 0.9 | 0.9 | 2.3 | |||
| Control group | Stress myocardial perfusion imaging | Standard of care | Standard of care | |||
| Randomization | 1:1 | 2:1 | 1:1 | |||
| Number of centres | 16 | 5 | 9 | |||
| Conventional Tn assays and thresholds used in the study | Tn I, Bayer, thresholds not reported | Not reported | Tn T, Roche: 0.03 ng/mL Tn I, Alere: 0.40 ng/mL Tn I, Beckman: 0.07/0.04 ng/mL |
|||
| Coronary CTA | Controls | Coronary CTA | Controls | Coronary CTA | Controls | |
| ACS during index hospitalization (%) | 1.2 | 2.7 | 4 | 2 | 9 | 6 |
| MACE during follow-up (%) | 0.8 | 0.4 | 3 | 1 | 0.4 | 1.2 |
| Time to diagnosis (h) | 2.9a | 6.2a | – | – | – | – |
| Length of stay (h) | – | – | 18.0a | 24.8a | 23.2a | 30.8a |
| Direct ED discharges (%) | – | – | 50a | 23a | 47a | 12a |
| Invasive coronary angiography (%) | 7 | 6 | 5 | 4 | 11 | 7 |
| Coronary revascularization (%) | 4 | 2 | 3 | 1 | 7 | 4 |
| ED cost ($) | 2137 | 3458 | – | – | 2101 | 2566 |
| Radiation dose (mSv) | 12 | 13 | – | – | 14a | 5a |
aSignificant difference between coronary CTA and control groups (P < 0.05).
Several keys exist to assist in improving the accuracy of hsTn testing for acute MI, helping to separate ‘coronary’ from ‘non-coronary’ causes of hsTn elevation.24 These include an understanding of the usual expected hsTn values associated with disorders that may lead to myonecrosis (Table 1), use of serial testing, and combining hsTn testing with clinical risk scoring. One specific area is hsTn in the setting of renal dysfunction and associated cardiac disease. While the levels elevated of hsTn can be observed in the population of patients with renal dysfunction, hsTn assays maintain high diagnostic accuracy for MI, particularly when significant rise and/or fall is observed. To ensure the best possible clinical use, higher assay-specific optimal cut-off levels might be considered.25
Table 1.
Possible non-acute coronary syndrome causes of troponin elevation
| Renal dysfunction and associated cardiac disease |
| Severe congestive heart failure—acute or chronic |
| Hypertensive crisis |
| Tachy- and brady-arrhythmia |
| Pulmonary embolism, severe pulmonary hypertension |
| Myocarditis |
| Acute neurological disease (stroke, subarachnoid haemorrhage) |
| Aortic dissection, aortic valve disease, or hypertrophic cardiomyopathy |
| Cardiac contusion, ablation, pacing, cardioversion, or endomyocardial biopsy |
| Hypothyroidism |
| Stress-induced cardiomyopathy |
| Infiltrative diseases, e.g. amyloidosis, haemochromatosis, sarcoidosis, and scleroderma |
| Drug toxicity, e.g. anthracyclines, Her-2 blockers, 5-fluorouracil, and snake venoms |
| Burns, if affecting >30% of body surface area |
| Rhabdomyolysis |
| Critically ill patients, especially with respiratory failure, or sepsis |
Modified from European Society of Cardiology guidelines.5
Assessment of highly sensitive troponin in patients with suspected acute coronary syndrome
While the great majority of patients tested in an ED setting have measureable hsTn concentrations, a certain percentage are below the limit of detection for the assay. Depending on the lower end sensitivity, being below the limit of detection may be associated with a high likelihood for an absence of ACS and/or acute MI.13,16,19,26 For example, in a large registry study of almost 15 000 patients, between 10 and 15% of patients had hsTn below the limit of detection and such patients were very low risk with a 0.44% MACE rate at 30 days.27 In another study, Body et al. showed that hsTn T below the limit of detection at arrival had a 100% negative predictive value for acute MI, allowing for exclusion of the diagnosis at first draw in this cohort in up to nearly 28%.15 Subsequently, other studies have confirmed and extended these results; in the Rule Out Myocardial Infarction/Ischemia Using Computer Assisted Tomography (ROMICAT) II trial, Januzzi et al. recently showed concentrations of hsTn I below the limit of detection not only excluded ACS (including unstable angina pectoris) with 100% negative predictive value, but also excluded significant coronary stenoses in those undergoing subsequent coronary CTA.19 Thus, when using hsTn methods, very low biomarker concentrations may be useful.
When employing serial testing, clinicians should understand that the demonstration of a significant increase (or decrease) in hsTn is usually indicative of an acute disease process, as opposed to an unchanging Tn result that is seen in chronic disease states.18,28 In this regard, a very low and stable value helps to exclude MI, while the detection of a substantial rise and/or fall of hsTn in a very short period of time may correctly identify acute MI in a large percentage of cases.
For example, in the Advantageous Predictors of Acute Coronary Syndromes Evaluation (APACE) study, Reichlin et al. studied patients with undifferentiated chest pain in the ED with hsTn measurements at the time of presentation and after 1 h.14 They derived the low hsTn T threshold (<12 ng/L) at the time of presentation and a small absolute change of hsTn T (<3 ng/L) at 1 h as criteria for rule out of ACS. They validated their results in an independent cohort and achieved 100% sensitivity and negative predictive value for the diagnosis of acute MI. In a subsequent analysis from the same cohort, Druey et al. showed that an accelerated protocol of hsTn I testing over a 1-h period correctly excluded acute MI in 65% of subjects with no change in hsTn I (with negative predictive value of 98.6%) and correctly identified acute MI in 12% when hsTn I was elevated or rising (with positive predictive value of 76.3%).29 In both analyses, however, a significant number of subjects were between the ‘rule-out’ and ‘rule-in’ categories, and would require observation and/or further testing.
Beyond accuracy for diagnosis, rapid ED protocols with two hsTn measurements at the time of presentation and after 1 or 2 h also allow for sensitivity and negative predictive value of >99% for 30-day MACE.14,18,30 Up to 40% of patients presenting to the ED with suspected ACS were classified as low risk in these studies and are potential candidates for early discharge from the ED and outpatient work-up.
As biomarker testing should never be used in isolation without clinical corroboration, studies have focussed on the development of accelerated diagnostic protocols (ADP) for ED triage. These protocols not only include information from serial hsTn testing but also add clinical information, namely electrocardiogram and clinical risk assessment such as the Thrombolysis in Myocardial Infarction (TIMI) risk score. For example, in the 2-Hour Accelerated Diagnostic Protocol to Assess Patients With Chest Pain Symptoms Using Contemporary Troponins as the Only Biomarker (ADAPT) study, Than et al. showed that among 1975 patients, incorporation of a TIMI risk score of 0 together with hsTn values below the 99th percentile value allowed for sensitivity of 99.7% and negative predictive value 99.7% for excluding risk for MACE.31 In a subsequent analysis, pooling the ADAPT and APACE trials, the investigators explored the negative predictive value of an ADP incorporating an electrocardiogram without ischaemic changes, TIMI score of 0 or ≤1 and serial hsTn <99th percentile at the time of presentation and after 2 h.18 The negative predictive value of the ADP triage with the threshold of TIMI score of 0- for 30-day MACE was 100%. When patients with TIMI score of 1 were included, the negative predictive value decreased to 99.7%. However, the proportion of patients who could be potentially discharged from the ED increased from 25 to 39%. The results for rapid ED protocols were confirmed with various hsTn assays.18,30
While the use of low concentrations of hsTn at presentation, reassuring serial measurements, and/or ADP-supported evaluation allow for prediction of very low 30-day MACE event rates and assist in more rapid discharge, risk may be still present in this patient group indicating the need for close outpatient follow-up. For patients with measurable (i.e. above the limit of detection) hsTn values, particularly those with a TIMI risk score >1, and/or those in the ‘observation’ categories in rapid serial testing protocols, further testing such as stress testing or coronary CTA will likely be needed.
Coronary computed tomography angiography in patients with suspected acute coronary syndrome
Coronary CTA has evolved in an alternative diagnostic testing strategy for patients with suspected ACS in the ED. The major strength of coronary CTA is its high negative predictive value for obstructive coronary artery disease (CAD). In the clinical practice, the majority of patients with ACS have obstructive CAD and the absence of significant stenosis significantly decreases the likelihood of ACS.32,33 The diagnostic performance of coronary CTA for the assessment of coronary stenosis has been demonstrated in many studies, among them several multicentre trials that compared coronary CTA with invasive coronary angiography in patients with stable chest pain (sensitivity between 85 and 99%, specificity between 64 and 97%, negative predictive value >95%).34–37 The performance of coronary CTA can be limited in patients with high heart rates and the presence of extensive coronary calcium.
Earlier single-centre studies of patients with suspected ACS demonstrated that the exclusion of a significant coronary stenosis by coronary CTA has high negative predictive value (>99%) for ACS and thus may potentially allow for earlier discharge than functional testing.38–50
Subsequently, three multicentre randomized trials were performed that compared coronary CTA with the standard evaluation of acute chest pain patients in the ED.40,46,51 The results from those studies that enrolled >3000 patients with low-to-intermediate likelihood of ACS are summarized in Table 2. The inclusion criteria included the ED presentation with low-to-intermediate likelihood of ACS, negative initial conventional Tn, and non-ischaemic electrocardiograms. Patients with previous history of CAD were excluded. In all three trials, the primary outcome of interest—the length of stay or time to diagnosis—was significantly shorter in the coronary CTA arm when compared with standard of care, which included stress testing in the majority of patients (Figure 4). The proportion of direct discharges increased approximately four times (50 vs. 12%).46,51 The increased efficiency of the ED triage was accomplished safely, without an increase in MACE during a 28-day follow-up.
Figure 4.
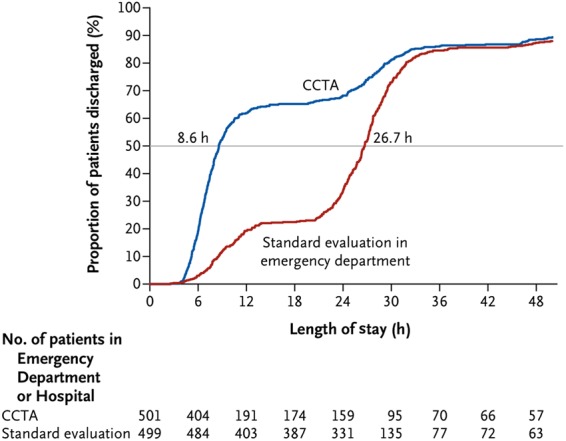
Length of stay and proportion of patients discharged in the Rule Out Myocardial Infarction/Ischemia Using Computer Assisted Tomography II trial. The cumulative frequency of discharges from the index visit according to the length of stay is shown. The horizontal line indicates the median length of stay in the two study groups, which was significantly different (8.6 h in the coronary computed tomography angiography vs. 26.7 h in the standard-evaluation group, P < 0.001). Reprinted with permission from Hoffmann et al.51
The downside of the coronary CTA use in the ED patients is a trend towards increased additional testing and invasive procedures.40,46,51 This trend is probably due to the increased sensitivity of coronary CTA for the detection of CAD and the overestimation of stenosis degree in routine clinical practice. The increase in invasive coronary angiography and percutaneous coronary interventions (PCI) after coronary CTA was estimated at 21 and 20 per 1000 scans, respectively.52 At this point, there are no reliable data to indicate whether the improved detection of CAD and subsequent PCI in this acute setting will improve long-term health outcomes. Despite the increase in subsequent testing and interventions, the overall cost of the index hospitalization was not significantly higher in those randomized to coronary CTA.51 Furthermore, lifelong Markov modelling from the ROMICAT II trial demonstrated cost-effective improvement of health outcomes when compared with functional testing with incremental cost-effectiveness ratio of $37 000 per quality-adjusted life-year.53
The estimated radiation exposure for those evaluated using coronary CTA may be a concern. In clinical trials, those randomized to coronary CTA had higher (13.9 mSv) radiation dose when compared with the standard of care (4.7 mSv), including patients undergoing only exercise treadmill stress test or stress echocardiography,51 but was similar in a direct comparison of coronary CTA (11.2 mSv) and stress myocardial perfusion imaging (12.8 mSv) in the Coronary Computed Tomographic Angiography for Systematic Triage of Acute Chest Pain Patients to Treatment (CT-STAT) trial.40 Some authors propose that these radiation doses are associated with increased lifelong risk of cancer, although there is an ongoing discussion about the carcinogenic effect of ionizing radiation used in medical imaging and direct epidemiological data confirming this effect are missing. The estimated life-time-associated risk of cancer was estimated to be between 1 in 100 and 1 in 3000 CT scans with estimated radiation dose of 9–21 mSv.54 The risk increases with younger age and is higher in women. With coronary CTA protocols now allowing for less radiation exposure (average dose of 3 mSv and low-dose scans performed with doses <1 mSv) without loss of diagnostic accuracy, this concern may be allayed.55
Novel approaches for the assessment of coronary computed tomography angiography
There is growing evidence that additional information obtained during coronary CTA such as the assessment of global and regional LV function, evaluation of myocardial perfusion, non-invasive fractional flow reserve (FFR), and coronary plaque analysis have the potential to improve the accuracy and efficiency of coronary CTA.
Evaluation of global and regional LV function is feasible with coronary CTA acquisition, which covers all phases of cardiac cycle, and the results compare favourably with echocardiography and cardiac magnetic resonance imaging.56,57 The analyses of ED patients showed that the presence of regional LV dysfunction incrementally and independently improved the diagnostic accuracy for ACS beyond stenosis detection in patients with the presence of CAD (sensitivity of coronary stenosis vs. coronary stenosis and LV dysfunction: 77 vs. 87%).58–60 This is specifically valuable in patients with non-diagnostic assessment for significant stenosis or borderline stenosis.
The evaluation of first-pass myocardial perfusion at rest is feasible with a standard coronary CTA acquisition.57 First-pass perfusion defects were observed in the majority of patients with MI, but less commonly in patients with unstable angina pectoris.61,62 Several small studies demonstrated that resting first-pass myocardial perfusion defects detected on coronary CTA increased specificity and positive predictive value for ACS (from 67 to 90%).59,63–65
The recent developments in computational fluid dynamics and image-based modelling now permit the determination of non-invasive FFR without the need for additional imaging, modification of coronary CTA acquisition protocols, or administration of medication (adenosine).66 Non-invasive FFR measurements have been shown to correlate with invasive FFR.67,68
In the Determination of Fractional Flow Reserve by Anatomic Computed Tomographic Angiography study, non-invasive FFR demonstrated sensitivity of 73% and specificity of 90% for the detection of per-patient ischaemia as determined by invasive FFR.68 The overall diagnostic accuracy of non-invasive FFR for per-patient detection of ischaemia was significantly better when compared with the detection of obstructive CAD by coronary CTA [area under the curve (AUC) 0.81 vs. 0.68, P < 0.001]. Similar results were reported in the Analysis of Coronary Blood Flow Using CT Angiography: Next Steps (NXT) study.69 Non-invasive FFR when compared with anatomic coronary CTA detection of significant stenosis improved the diagnostic accuracy for the detection of lesion-specific ischaemia with the improvement of specificity from 34 to 79% and overall accuracy (AUC 0.90 vs. 0.81, P < 0.001). Furthermore, the addition of FFR to the traditional assessment for obstructive CAD improved specificity for the detection of ischaemia and diagnostic accuracy in patients with intermediate stenoses (40–69%).70 The addition of FFR to the assessment of coronary CTA may improve the sensitivity and specificity of the test and correctly identify patients with haemodynamically significant CAD with lesion-specific ischaemia in the ED. However, no studies have been performed in a population of patients with acute chest pain presentations up to date.
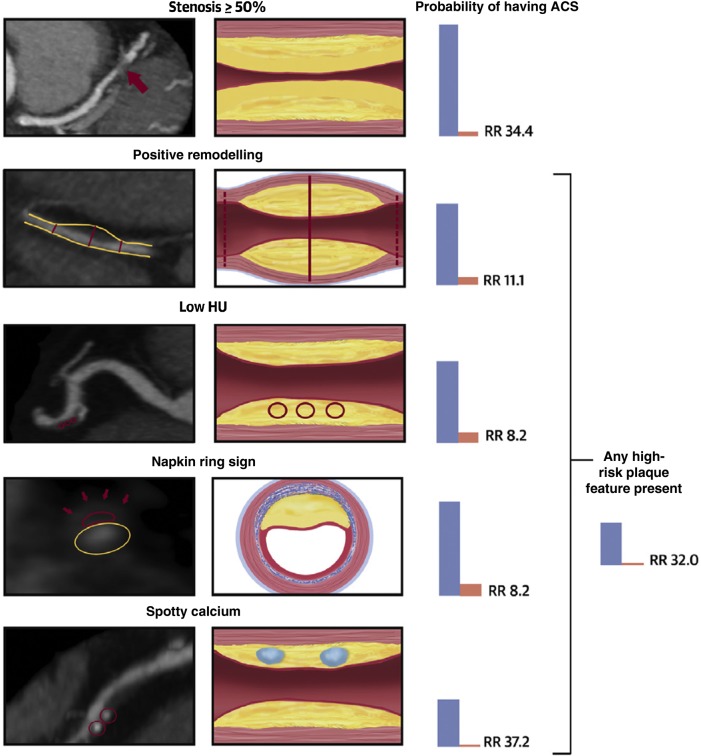
Feasibility of coronary plaque analysis using coronary CTA data has been well established.71–73 Coronary CTA permits assessment of plaque composition based on CT attenuation and detection of high-risk plaque features such as positive remodelling, percentage of plaque burden, low CT attenuation plaque, napkin-ring sign, and spotty calcium.72,74–81 High-risk plaque features were more often seen in culprit coronary lesions of ACS than in stenotic lesions in stable angina pectoris patients.82–87 Furthermore, the presence of high-risk plaque was independent of significant stenosis for the detection of ischaemia as measured by invasive FFR.88 In the ROMICAT I trial, patients with acute chest pain presentation and with the presence of ≥50% stenosis could be differentiated into those with and without ACS using a coronary plaque score, which included spotty calcium, low CT attenuation, positive remodelling, and length of stenosis.89 More recently, the analysis of almost 500 patients randomized to the coronary CTA arm of the ROMICAT II trial showed that the presence of high-risk plaque features (positive remodelling, low CT attenuation plaque with <30 Hounsfield units, napkin-ring sign, and spotty calcium) was independent and incremental to ≥50% stenosis for the diagnosis of ACS (Figure 5).90 Overall, there is an increasing evidence that advanced plaque characterization may provide incremental value for the management of acute chest pain patients, specifically identifying those whose symptoms may not be associated with significant CAD found ‘incidentally’ on coronary CTA and those without significant CAD who may be at increased risk for ACS.
Figure 5.
The association of significant stenosis and high-risk plaque features with the probability of acute coronary syndrome in the Rule Out Myocardial Infarction/Ischemia Using Computer Assisted Tomography II trial. Stenosis ≥50%: severe stenosis of the mid-left anterior descending coronary artery (red arrow). Positive remodelling: non-calcified plaque with positive remodelling in the distal right coronary artery. The two-dotted red lines demonstrate the vessel diameters at the proximal and distal references (both 1.8 mm), and the solid red line demonstrates the maximal vessel diameter in the mid-portion of the plaque (2.7 mm). The remodelling index is 1.5. Low Hounsfield units plaque: partially calcified plaque in the mid-right coronary artery with low <30 Hounsfield unit plaque. The red circles demonstrate the three regions of interest, with mean computed tomography numbers of 22, 19, and 20 Hounsfield units. Napkin-ring sign: napkin-ring sign plaque in the mid-left anterior descending coronary artery. Schematic cross-sectional view of the napkin-ring sign. The red line demonstrates the central low Hounsfield unit area of the plaque adjacent to the lumen (yellow ellipse) surrounded by a peripheral rim of the higher computed tomography attenuation (red arrows). Spotty calcium: partially calcified plaque in the mid-right coronary artery with spotty calcification (diameter <3 mm in all directions; red circles). Reprinted with permission from Puchner et al.90
Combined use of highly sensitive troponin and coronary computed tomography angiography
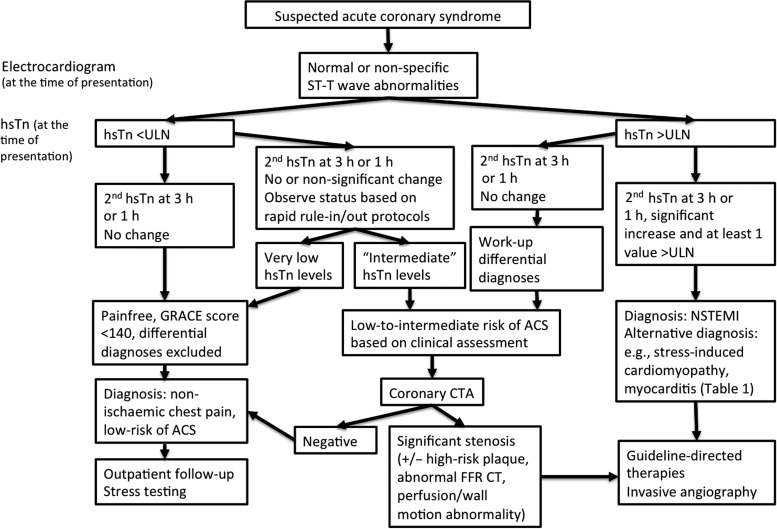
Based on the presented data for hsTn and coronary CTA, the combined approach of these two diagnostic tests may permit rapid evaluation and triage of the majority of ED patients with suspected ACS (Figure 6). In Europe, recently updated guidelines include rapid hsTn rule-out and rule-in protocols and significantly decrease the proportion of patients who require further work-up.5 Nevertheless, there is a subgroup of patients that even with hsTn will require observation status and rapid coronary CTA will permit early discharge. Furthermore, there are patients with mildly elevated or ‘intermediate’ hsTn without typical rise and fall, who may also benefit from coronary CTA.
Figure 6.
Possible implementation of highly sensitive troponin and coronary computed tomography angiography in the evaluation of patients with suspected acute coronary syndrome in the emergency department.
We performed a retrospective analysis in a subset of 160 patients from the ROMICAT II trial who had blood samples available and underwent coronary CTA. Highly sensitive Tn I (hsVista, Siemens) below the limit of detection at the time of presentation had negative predictive value of 100% for ACS during the index hospitalization and would permit rapid discharge of 6% patients without further testing.91 Patients with hsTn I >99th percentile represented a high-risk group with ACS event rate of 58%. Patients with intermediate hsTn I levels at the time of presentation (above the limit of detection, but <99th percentile) had an intermediate risk of ACS with an event rate of 9%. Coronary CTA could reclassify the risk of ACS in this group. Among those patients with measurable but not elevated hsTn I and with no evidence of significant stenosis or high-risk plaque (54%), none had ACS (negative predictive value of 100%), whereas those with measurable but not elevated hsTn I with significant stenosis and high-risk plaque had high risk of ACS (event rate 69%). In summary, 60% of patients (hsTn below the limit of detection or negative coronary CTA) could be possibly discharged after initial hsTn and coronary CTA, usually within 2 h of their presentation to the ED. On the other hand, additional 16% of patients (hsTn >99% percentile or positive coronary CTA) could be categorized as highest risk and receive appropriate therapies early in the course of their hospitalization. The combined use of hsTn and coronary CTA may result in cost-saving and decreased radiation exposure by decreasing the need for additional advanced cardiac testing in patients with hsTn below the limit of detection. Additionally, among those safely discharged from the ED but with anatomic definition of their coronary arteries, knowledge of the presence of previously unsuspected sub-critical CAD should allow for aggressive application of secondary prevention measures, such as more directed use of statins or anti-platelet agents.
There is an increased interest in the rapid rule-out protocols with clinical assessment followed by hsTn and discharge to outpatient follow-up if the risk of ACS is low. The advantages of rapid protocols are decreased time to discharge and cost-efficiency. However, there is still a need for outpatient testing, which may be challenging, and follow-up varies significantly among various medical systems and countries. In addition, coronary CTA provides prolonged warranty period beyond the initial ED presentation with the evidence of virtually no MACE in patients with no evidence of coronary atherosclerosis for ∼2 years; thus, possibly decreasing the need for testing during subsequent ED presentations.92 Furthermore, coronary atherosclerosis is detected in approximately half of patients with suspected ACS in the ED by coronary CTA and would not be detected with rapid rule-out protocols.40,46,51 This may have significant consequences for the outpatient management and preventative therapies. Whether the appropriate preventative strategies, guided by coronary CTA finding, will lead to improved outcomes will need to be studied. In summary, we believe that combination of hsTn and coronary CTA in the ED patients with suspected ACS is a valuable concept that should be tested in a well-designed randomized trial.
Conclusions
Highly sensitive Tn assays are an established tool for the rapid and efficient triage of ED patients with suspected ACS. Coronary CTA is a promising diagnostic tool, which in a subset of patients may facilitate early discharge. It will be critical to educate clinicians about the advantages of both techniques with a specific emphasis on the interpretation of the results on probabilistic basis rather than as dichotomous values (i.e. positive vs. negative test). The results of both tests (especially in those with intermediate results) have implications for outpatient management and preventative strategies and need to be effectively communicated to primary care providers.
Authors’ contributions
M.F., U.H., F.B., and J.L.J. handled funding and supervision, acquired the data, conceived and designed the research, drafted the manuscript, and made critical revision of the manuscript for key intellectual content.
Conflict of interest: M.F.: research grant/significant: American Heart Association Fellow to Faculty Award 13FTF16450001. U.H.: research grant/significant: NIH U01HL092040, U01HL092022, Siemens Medical Solutions, and Heart Flow Inc.; consultant/advisory board/significant: Heart Flow. F.B.: unrestricted research grant: Bayer Healthcare (Berlin, Germany) and Siemens Healthcare (Forchheim, Germany); speakers bureau: Bayer Healthcare (Berlin, Germany) and Siemens Healthcare (Forchheim, Germany). J.L.J.: research grant/significant: Siemens, Thermo Fisher, and Singulex; consultant/advisory board/significant: Critical Diagnostics, Sphingotec, and Roche.
References
- 1. Niska R, Bhuiya F, Xu J. National Hospital Ambulatory Medical Care Survey: 2007 emergency department summary. Natl Health Stat Report 2010;1–31. Report No. 26. [PubMed] [Google Scholar]
- 2. McCaig LF, Burt CW. National Hospital Ambulatory Medical Care Survey: 2002 Emergency Department Summary. Advanced Data from Vital and Health Statistics. National Center for Health Statistics; 2004. pp. 1–36. Report No.: 340. [PubMed]
- 3. Pope JH, Aufderheide TP, Ruthazer R, Woolard RH, Feldman JA, Beshansky JR, Griffith JL, Selker HP. Missed diagnoses of acute cardiac ischemia in the emergency department. N Engl J Med 2000;342:1163–1170. [DOI] [PubMed] [Google Scholar]
- 4. Swap CJ, Nagurney JT. Value and limitations of chest pain history in the evaluation of patients with suspected acute coronary syndromes. JAMA 2005;294:2623–2629. [DOI] [PubMed] [Google Scholar]
- 5. Authors/Task Force Members Roffi M, Patrono C, Collet J-P, Mueller C, Valgimigli M, Andreotti F, Bax JJ, Borger MA, Brotons C, Chew DP, Gencer B, Hasenfuss G, Kjeldsen K, Lancellotti P, Landmesser U, Mehilli J, Mukherjee D, Storey RF, Windecker S. 2015 ESC guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation: Task Force for the Management of Acute Coronary Syndromes in Patients Presenting without Persistent ST-Segment Elevation of the European Society of Cardiology (ESC). Eur Heart J 2015;1–59. [Epub ahead of print].25567812 [Google Scholar]
- 6. Anderson JL, Adams CD, Antman EM, Bridges CR, Califf RM, Casey DE, Chavey WE, Fesmire FM, Hochman JS, Levin TN, Lincoff AM, Peterson ED, Theroux P, Wenger NK, Wright RS, Smith SC. 2011 ACCF/AHA focused update incorporated into the ACC/AHA 2007 guidelines for the management of patients with unstable angina/non-ST-elevation myocardial infarction: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation 2011;123:e426–e579. [DOI] [PubMed] [Google Scholar]
- 7. Thygesen K, Alpert JS, Jaffe AS, Simoons ML, Chaitman BR, White HD, Joint ESC/ACCF/AHA/WHF Task Force for Universal Definition of Myocardial Infarction, Authors/Task Force Members Chairpersons, Thygesen K, Alpert JS, White HD, Biomarker Subcommittee, Jaffe AS, Katus HA, Apple FS, Lindahl B, Morrow DA, ECG Subcommittee, Chaitman BR, Clemmensen PM, Johanson P, Hod H, Imaging Subcommittee, Underwood R, Bax JJ, Bonow JJ, Pinto F, Gibbons RJ, Classification Subcommittee, Fox KA, et al. Third universal definition of myocardial infarction. J Am Coll Cardiol 2012;60:1581–1598.22958960 [Google Scholar]
- 8. Apple FS, Collinson PO, IFCC Task Force on Clinical Applications of Cardiac Biomarkers. Analytical characteristics of high-sensitivity cardiac troponin assays. Clin Chem 2012;58:54–61. [DOI] [PubMed] [Google Scholar]
- 9. Thygesen K, Mair J, Giannitsis E, Mueller C, Lindahl B, Blankenberg S, Huber K, Plebani M, Biasucci LM, Tubaro M, Collinson P, Venge P, Hasin Y, Galvani M, Koenig W, Hamm C, Alpert JS, Katus H, Jaffe AS, Study Group on Biomarkers in Cardiology of ESC Working Group on Acute Cardiac Care. How to use high-sensitivity cardiac troponins in acute cardiac care. Eur Heart J 2012;33:2252–2257. [DOI] [PubMed] [Google Scholar]
- 10. de Lemos JA, Drazner MH, Omland T, Ayers CR, Khera A, Rohatgi A, Hashim I, Berry JD, Das SR, Morrow DA, McGuire DK. Association of troponin T detected with a highly sensitive assay and cardiac structure and mortality risk in the general population. JAMA 2010;304:2503–2512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Saunders JT, Nambi V, de Lemos JA, Chambless LE, Virani SS, Boerwinkle E, Hoogeveen RC, Liu X, Astor BC, Mosley TH, Folsom AR, Heiss G, Coresh J, Ballantyne CM. Cardiac troponin T measured by a highly sensitive assay predicts coronary heart disease, heart failure, and mortality in the Atherosclerosis Risk in Communities Study. Circulation 2011;123:1367–1376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Wang TJ, Wollert KC, Larson MG, Coglianese E, McCabe EL, Cheng S, Ho JE, Fradley MG, Ghorbani A, Xanthakis V, Kempf T, Benjamin EJ, Levy D, Vasan RS, Januzzi JL. Prognostic utility of novel biomarkers of cardiovascular stress: the Framingham Heart Study. Circulation 2012;126:1596–1604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Reichlin T, Hochholzer W, Bassetti S, Steuer S, Stelzig C, Hartwiger S, Biedert S, Schaub N, Buerge C, Potocki M, Noveanu M, Breidthardt T, Twerenbold R, Winkler K, Bingisser R, Mueller C. Early diagnosis of myocardial infarction with sensitive cardiac troponin assays. N Engl J Med 2009;361:858–867. [DOI] [PubMed] [Google Scholar]
- 14. Reichlin T, Schindler C, Drexler B, Twerenbold R, Reiter M, Zellweger C, Moehring B, Ziller R, Hoeller R, Rubini Giménez M, Haaf P, Potocki M, Wildi K, Balmelli C, Freese M, Stelzig C, Freidank H, Osswald S, Mueller C. One-hour rule-out and rule-in of acute myocardial infarction using high-sensitivity cardiac troponin T. Arch Intern Med 2012;172:1211–1218. [DOI] [PubMed] [Google Scholar]
- 15. Body R, Carley S, McDowell G, Jaffe AS, France M, Cruickshank K, Wibberley C, Nuttall M, Mackway-Jones K. Rapid exclusion of acute myocardial infarction in patients with undetectable troponin using a high-sensitivity assay. J Am Coll Cardiol 2011;58:1332–1339. [DOI] [PubMed] [Google Scholar]
- 16. Keller T, Zeller T, Peetz D, Tzikas S, Roth A, Czyz E, Bickel C, Baldus S, Warnholtz A, Fröhlich M, Sinning CR, Eleftheriadis MS, Wild PS, Schnabel RB, Lubos E, Jachmann N, Genth-Zotz S, Post F, Nicaud V, Tiret L, Lackner KJ, Münzel TF, Blankenberg S. Sensitive troponin I assay in early diagnosis of acute myocardial infarction. N Engl J Med 2009;361:868–877. [DOI] [PubMed] [Google Scholar]
- 17. Keller T, Zeller T, Ojeda F, Tzikas S, Lillpopp L, Sinning C, Wild P, Genth-Zotz S, Warnholtz A, Giannitsis E, Möckel M, Bickel C, Peetz D, Lackner K, Baldus S, Münzel T, Blankenberg S. Serial changes in highly sensitive troponin I assay and early diagnosis of myocardial infarction. J Am Med Assoc 2011;306:2684–2693. [DOI] [PubMed] [Google Scholar]
- 18. Cullen L, Mueller C, Parsonage WA, Wildi K, Greenslade JH, Twerenbold R, Aldous S, Meller B, Tate JR, Reichlin T, Hammett CJ, Zellweger C, Ungerer JP, Rubini Gimenez M, Troughton R, Murray K, Brown AF, Mueller M, George P, Mosimann T, Flaws DF, Reiter M, Lamanna A, Haaf P, Pemberton CJ, Richards AM, Chu K, Reid CM, Peacock WF, Jaffe AS, Florkowski C, Deely JM, Than M. Validation of high-sensitivity troponin I in a 2-hour diagnostic strategy to assess 30-day outcomes in emergency department patients with possible acute coronary syndrome. J Am Coll Cardiol 2013;62:1242–1249. [DOI] [PubMed] [Google Scholar]
- 19. Januzzi JL, Sharma U, Zakroysky P, Truong QA, Woodard PK, Pope JH, Hauser T, Mayrhofer T, Nagurney JT, Schoenfeld D, Peacock WF, Fleg JL, Wiviott S, Pang PS, Udelson J, Hoffmann U. Sensitive troponin assays in patients with suspected acute coronary syndrome: results from the multicenter rule out myocardial infarction using computer assisted tomography II trial. Am Heart J 2015;169:572–578.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Januzzi JL, Bamberg F, Lee H, Truong QA, Nichols JH, Karakas M, Mohammed AA, Schlett CL, Nagurney JT, Hoffmann U, Koenig W. High-sensitivity troponin T concentrations in acute chest pain patients evaluated with cardiac computed tomography. Circulation 2010;121:1227–1234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Januzzi JL, Filippatos G, Nieminen M, Gheorghiade M. Troponin elevation in patients with heart failure: on behalf of the third Universal Definition of Myocardial Infarction Global Task Force: Heart Failure Section. Eur Heart J 2012;33:2265–2271. [DOI] [PubMed] [Google Scholar]
- 22. Jaffe AS, Apple FS, Morrow DA, Lindahl B, Katus HA. Being rational about (im)precision: a statement from the Biochemistry Subcommittee of the Joint European Society of Cardiology/American College of Cardiology Foundation/American Heart Association/World Heart Federation Task Force for the definition of myocardial infarction. Clin Chem 2010;56:941–943. [DOI] [PubMed] [Google Scholar]
- 23. Wildi K, Gimenez MR, Twerenbold R, Reichlin T, Jaeger C, Heinzelmann A, Arnold C, Nelles B, Druey S, Haaf P, Hillinger P, Schaerli N, Kreutzinger P, Tanglay Y, Herrmann T, Moreno Weidmann Z, Krivoshei L, Freese M, Stelzig C, Puelacher C, Rentsch K, Osswald S, Mueller C. Misdiagnosis of myocardial infarction related to limitations of the current regulatory approach to define clinical decision values for cardiac troponin. Circulation 2015;131:2032–2040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Januzzi JL. What to expect when measuring high-sensitivity troponin: practical advice for clinicians. J Am Coll Cardiol 2015;65:1665–1667. [DOI] [PubMed] [Google Scholar]
- 25. Twerenbold R, Wildi K, Jaeger C, Gimenez MR, Reiter M, Reichlin T, Walukiewicz A, Gugala M, Krivoshei L, Marti N, Moreno Weidmann Z, Hillinger P, Puelacher C, Rentsch K, Honegger U, Schumacher C, Zurbriggen F, Freese M, Stelzig C, Campodarve I, Bassetti S, Osswald S, Mueller C. Optimal cutoff levels of more sensitive cardiac troponin assays for the early diagnosis of myocardial infarction in patients with renal dysfunction. Circulation 2015;131:2041–2050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Rubini Giménez M, Hoeller R, Reichlin T, Zellweger C, Twerenbold R, Reiter M, Moehring B, Wildi K, Mosimann T, Mueller M, Meller B, Hochgruber T, Ziller R, Sou SM, Murray K, Sakarikos K, Ernst S, Gea J, Campodarve I, Vilaplana C, Haaf P, Steuer S, Minners J, Osswald S, Mueller C. Rapid rule out of acute myocardial infarction using undetectable levels of high-sensitivity cardiac troponin. Int J Cardiol 2013;168:3896–3901. [DOI] [PubMed] [Google Scholar]
- 27. Bandstein N, Ljung R, Johansson M, Holzmann MJ. Undetectable high-sensitivity cardiac troponin T level in the emergency department and risk of myocardial infarction. J Am Coll Cardiol 2014;63:2569–2578. [DOI] [PubMed] [Google Scholar]
- 28. Wu AHB, Bolger AF, Hollander JE. Growing pains with the use of high-sensitivity cardiac troponin assays. J Am Coll Cardiol 2013;62:1250–1251. [DOI] [PubMed] [Google Scholar]
- 29. Druey S, Wildi K, Twerenbold R, Jaeger C, Reichlin T, Haaf P, Rubini Giménez M, Puelacher C, Wagener M, Radosavac M, Honegger U, Schumacher C, Delfine V, Kreutzinger P, Herrmann T, Moreno Weidmann Z, Krivoshei L, Freese M, Stelzig C, Isenschmid C, Bassetti S, Rentsch K, Osswald S, Mueller C. Early rule-out and rule-in of myocardial infarction using sensitive cardiac Troponin I. Int J Cardiol 2015;195:163–170. [DOI] [PubMed] [Google Scholar]
- 30. Meller B, Cullen L, Parsonage WA, Greenslade JH, Aldous S, Reichlin T, Wildi K, Twerenbold R, Jaeger C, Hillinger P, Haaf P, Puelacher C, Kern V, Rentsch K, Stallone F, Rubini Giménez M, Ballarino P, Bassetti S, Walukiewicz A, Troughton R, Pemberton CJ, Richards AM, Chu K, Reid CM, Than M, Mueller C. Accelerated diagnostic protocol using high-sensitivity cardiac troponin T in acute chest pain patients. Int J Cardiol 2015;184:208–215. [DOI] [PubMed] [Google Scholar]
- 31. Than M, Cullen L, Aldous S, Parsonage WA, Reid CM, Greenslade J, Flaws D, Hammett CJ, Beam DM, Ardagh MW, Troughton R, Brown AFT, George P, Florkowski CM, Kline JA, Peacock WF, Maisel AS, Lim SH, Lamanna A, Richards AM. 2-Hour accelerated diagnostic protocol to assess patients with chest pain symptoms using contemporary troponins as the only biomarker: the ADAPT trial. J Am Coll Cardiol 2012;59:2091–2098. [DOI] [PubMed] [Google Scholar]
- 32. Diver DJ, Bier JD, Ferreira PE, Sharaf BL, McCabe C, Thompson B, Chaitman B, Williams DO, Braunwald E. Clinical and arteriographic characterization of patients with unstable angina without critical coronary arterial narrowing (from the TIMI-IIIA Trial). Am J Cardiol 1994;74:531–537. [DOI] [PubMed] [Google Scholar]
- 33. Roe MT, Harrington RA, Prosper DM, Pieper KS, Bhatt DL, Lincoff AM, Simoons ML, Akkerhuis M, Ohman EM, Kitt MM, Vahanian A, Ruzyllo W, Karsch K, Califf RM, Topol EJ. Clinical and therapeutic profile of patients presenting with acute coronary syndromes who do not have significant coronary artery disease. Circulation 2000;102:1101–1106. [DOI] [PubMed] [Google Scholar]
- 34. Miller JM, Rochitte CE, Dewey M, Arbab-Zadeh A, Niinuma H, Gottlieb I, Paul N, Clouse ME, Shapiro EP, Hoe J, Lardo AC, Bush DE, de Roos A, Cox C, Brinker J, Lima JAC. Diagnostic performance of coronary angiography by 64-row CT. N Engl J Med 2008;359:2324–2336. [DOI] [PubMed] [Google Scholar]
- 35. Budoff MJ, Dowe D, Jollis JG, Gitter M, Sutherland J, Halamert E, Scherer M, Bellinger R, Martin A, Benton R, Delago A, Min JK. Diagnostic performance of 64-multidetector row coronary computed tomographic angiography for evaluation of coronary artery stenosis in individuals without known coronary artery disease: results from the prospective multicenter ACCURACY (Assessment by Coronary Computed Tomographic Angiography of Individuals Undergoing Invasive Coronary Angiography) trial. J Am Coll Cardiol 2008;52:1724–1732. [DOI] [PubMed] [Google Scholar]
- 36. Meijboom WB, Meijs MFL, Schuijf JD, Cramer MJ, Mollet NR, van Mieghem CAG, Nieman K, van Werkhoven JM, Pundziute G, Weustink AC, de Vos AM, Pugliese F, Rensing B, Jukema JW, Bax JJ, Prokop M, Doevendans PA, Hunink MGM, Krestin GP, de Feyter PJ. Diagnostic accuracy of 64-slice computed tomography coronary angiography: a prospective, multicenter, multivendor study. J Am Coll Cardiol 2008;52:2135–2144. [DOI] [PubMed] [Google Scholar]
- 37. Chow BJW, Freeman MR, Bowen JM, Levin L, Hopkins RB, Provost Y, Tarride J-E, Dennie C, Cohen EA, Marcuzzi D, Iwanochko R, Moody AR, Paul N, Parker JD, O'Reilly DJ, Xie F, Goeree R. Ontario multidetector computed tomographic coronary angiography study: field evaluation of diagnostic accuracy. Arch Intern Med 2011;171:1021–1029. [DOI] [PubMed] [Google Scholar]
- 38. Rubinshtein R, Halon DA, Gaspar T, Jaffe R, Karkabi B, Flugelman MY, Kogan A, Shapira R, Peled N, Lewis BS. Usefulness of 64-slice cardiac computed tomographic angiography for diagnosing acute coronary syndromes and predicting clinical outcome in emergency department patients with chest pain of uncertain origin. Circulation 2007;115:1762–1768. [DOI] [PubMed] [Google Scholar]
- 39. Goldstein JA, Gallagher MJ, O'Neill WW, Ross MA, O'Neil BJ, Raff GL. A randomized controlled trial of multi-slice coronary computed tomography for evaluation of acute chest pain. J Am Coll Cardiol 2007;49:863–871. [DOI] [PubMed] [Google Scholar]
- 40. Goldstein JA, Chinnaiyan KM, Abidov A, Achenbach S, Berman DS, Hayes SW, Hoffmann U, Lesser JR, Mikati IA, O'Neil BJ, Shaw LJ, Shen MYH, Valeti US, Raff GL. The CT-STAT (Coronary Computed Tomographic Angiography for Systematic Triage of Acute Chest Pain Patients to Treatment) trial. J Am Coll Cardiol 2011;58:1414–1422. [DOI] [PubMed] [Google Scholar]
- 41. Chang S-A, Choi SI, Choi E-K, Kim H-K, Jung J-W, Chun EJ, Kim K-S, Cho Y-S, Chung W-Y, Youn T-J, Chae I-H, Choi D-J, Chang H-J. Usefulness of 64-slice multidetector computed tomography as an initial diagnostic approach in patients with acute chest pain. Am Heart J 2008;156:375–383. [DOI] [PubMed] [Google Scholar]
- 42. Hoffmann U, Nagurney JT, Moselewski F, Pena A, Ferencik M, Chae CU, Cury RC, Butler J, Abbara S, Brown DF, Manini A, Nichols JH, Achenbach S, Brady TJ. Coronary multidetector computed tomography in the assessment of patients with acute chest pain. Circulation 2006;114:2251–2260. [DOI] [PubMed] [Google Scholar]
- 43. Hoffmann U, Bamberg F, Chae CU, Nichols JH, Rogers IS, Seneviratne SK, Truong QA, Cury RC, Abbara S, Shapiro MD, Moloo J, Butler J, Ferencik M, Lee H, Jang I-K, Parry BA, Brown DF, Udelson JE, Achenbach S, Brady TJ, Nagurney JT. Coronary computed tomography angiography for early triage of patients with acute chest pain: the ROMICAT (Rule Out Myocardial Infarction using Computer Assisted Tomography) trial. J Am Coll Cardiol 2009;53:1642–1650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Marwan M, Pflederer T, Schepis T, Seltmann M, Klinghammer L, Muschiol G, Ropers D, Daniel WG, Achenbach S. Accuracy of dual-source CT to identify significant coronary artery disease in patients with uncontrolled hypertension presenting with chest pain: comparison with coronary angiography. Int J Cardiovasc Imaging 2012;28:1173–1180. [DOI] [PubMed] [Google Scholar]
- 45. Hollander JE, Chang AM, Shofer FS, McCusker CM, Baxt WG, Litt HI. Coronary computed tomographic angiography for rapid discharge of low-risk patients with potential acute coronary syndromes. Ann Emerg Med 2009;53:295–304. [DOI] [PubMed] [Google Scholar]
- 46. Litt HI, Gatsonis C, Snyder B, Singh H, Miller CD, Entrikin DW, Leaming JM, Gavin LJ, Pacella CB, Hollander JE. CT angiography for safe discharge of patients with possible acute coronary syndromes. N Engl J Med 2012;366:1393–1403. [DOI] [PubMed] [Google Scholar]
- 47. Hansen M, Ginns J, Seneviratne S, Slaughter R, Premaranthe M, Samardhi H, Harker J, Lai T, Walters DL, Bett N. The value of dual-source 64-slice CT coronary angiography in the assessment of patients presenting to an acute chest pain service. Heart Lung Circ 2010;19:213–218. [DOI] [PubMed] [Google Scholar]
- 48. Nasis A, Meredith IT, Nerlekar N, Cameron JD, Antonis PR, Mottram PM, Leung MC, Troupis JM, Crossett M, Kambourakis AG, Braitberg G, Hoffmann U, Seneviratne SK. Acute chest pain investigation: utility of cardiac CT angiography in guiding troponin measurement. Radiology 2011;260:381–389. [DOI] [PubMed] [Google Scholar]
- 49. Chow BJW, Joseph P, Yam Y, Kass M, Chen L, Beanlands RS, Ruddy TD. Usefulness of computed tomographic coronary angiography in patients with acute chest pain with and without high-risk features. Am J Cardiol 2010;106:463–469. [DOI] [PubMed] [Google Scholar]
- 50. Cury RC, Feuchtner GM, Batlle JC, Peña CS, Janowitz W, Katzen BT, Ziffer JA. Triage of patients presenting with chest pain to the emergency department: implementation of coronary CT angiography in a large urban health care system. AJR Am J Roentgenol 2013;200:57–65. [DOI] [PubMed] [Google Scholar]
- 51. Hoffmann U, Truong QA, Schoenfeld DA, Chou ET, Woodard PK, Nagurney JT, Pope JH, Hauser TH, White CS, Weiner SG, Kalanjian S, Mullins ME, Mikati I, Peacock WF, Zakroysky P, Hayden D, Goehler A, Lee H, Gazelle GS, Wiviott SD, Fleg JL, Udelson JE, ROMICAT-II Investigators. Coronary CT angiography versus standard evaluation in acute chest pain. N Engl J Med 2012;367:299–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Hulten E, Pickett C, Bittencourt MS, Villines TC, Petrillo S, Di Carli MF, Blankstein R. Outcomes after coronary computed tomography angiography in the emergency department: a systematic review and meta-analysis of randomized, controlled trials. J Am Coll Cardiol 2013;61:880–892. [DOI] [PubMed] [Google Scholar]
- 53. Goehler A, Mayrhofer T, Pursnani A, Lumish H, Barth C, Nagurney JT, Truong QA, McMahon P, Gazelle SG, Hoffmann U. Comparison of long term health and economic outcomes of ED triage strategies for patients with acute chest pain. Circulation 2013;128(suppl.):A18295. [Google Scholar]
- 54. Einstein AJ, Henzlova MJ, Rajagopalan S. Estimating risk of cancer associated with radiation exposure from 64-slice computed tomography coronary angiography. JAMA 2007;298:317–323. [DOI] [PubMed] [Google Scholar]
- 55. Engel L-C, Lee AM, Seifarth H, Sidhu MS, Brady TJ, Hoffmann U, Ghoshhajra BB. Weekly dose reports: the effects of a continuous quality improvement initiative on coronary computed tomography angiography radiation doses at a tertiary medical center. Acad Radiol 2013;20:1015–1023. [DOI] [PubMed] [Google Scholar]
- 56. Greupner J, Zimmermann E, Grohmann A, Dübel H-P, Althoff TF, Althoff T, Borges AC, Rutsch W, Schlattmann P, Hamm B, Dewey M. Head-to-head comparison of left ventricular function assessment with 64-row computed tomography, biplane left cineventriculography, and both 2- and 3-dimensional transthoracic echocardiography: comparison with magnetic resonance imaging as the reference standard. J Am Coll Cardiol 2012;59:1897–1907. [DOI] [PubMed] [Google Scholar]
- 57. Cury RC, Nieman K, Shapiro MD, Butler J, Nomura CH, Ferencik M, Hoffmann U, Abbara S, Jassal DS, Yasuda T, Gold HK, Jang I-K, Brady TJ. Comprehensive assessment of myocardial perfusion defects, regional wall motion, and left ventricular function by using 64-section multidetector CT. Radiology 2008;248:466–475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Seneviratne SK, Truong QA, Bamberg F, Rogers IS, Shapiro MD, Schlett CL, Chae CU, Cury R, Abbara S, Brady TJ, Nagurney JT, Hoffmann U. Incremental diagnostic value of regional left ventricular function over coronary assessment by cardiac computed tomography for the detection of acute coronary syndrome in patients with acute chest pain: from the ROMICAT trial. Circ Cardiovasc Imaging 2010;3:375–383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Bezerra HG, Loureiro R, Irlbeck T, Bamberg F, Schlett CL, Rogers I, Blankstein R, Truong QA, Brady TJ, Cury RC, Hoffmann U. Incremental value of myocardial perfusion over regional left ventricular function and coronary stenosis by cardiac CT for the detection of acute coronary syndromes in high-risk patients: a subgroup analysis of the ROMICAT trial. J Cardiovasc Comput Tomogr 2011;5:382–391. [DOI] [PubMed] [Google Scholar]
- 60. Dirksen MS, Jukema JW, Bax JJ, Lamb HJ, Boersma E, Tuinenburg JC, Geleijns J, van der Wall EE, de Roos A. Cardiac multidetector-row computed tomography in patients with unstable angina. Am J Cardiol 2005;95:457–461. [DOI] [PubMed] [Google Scholar]
- 61. Schepis T, Achenbach S, Marwan M, Muschiol G, Ropers D, Daniel WG, Pflederer T. Prevalence of first-pass myocardial perfusion defects detected by contrast-enhanced dual-source CT in patients with non-ST segment elevation acute coronary syndromes. Eur Radiol 2010;20:1607–1614. [DOI] [PubMed] [Google Scholar]
- 62. Busch JL, Alessio AM, Caldwell JH, Gupta M, Mao S, Kadakia J, Shuman W, Budoff MJ, Branch KR. Myocardial hypo-enhancement on resting computed tomography angiography images accurately identifies myocardial hypoperfusion. J Cardiovasc Comput Tomogr 2011;5:412–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Feuchtner GM, Plank F, Pena C, Battle J, Min J, Leipsic J, Labounty T, Janowitz W, Katzen B, Ziffer J, Cury RC. Evaluation of myocardial CT perfusion in patients presenting with acute chest pain to the emergency department: comparison with SPECT-myocardial perfusion imaging. Heart 2012;98:1510–1517. [DOI] [PubMed] [Google Scholar]
- 64. Branch KR, Busey J, Mitsumori LM, Strote J, Caldwell JH, Busch JH, Shuman WP. Diagnostic performance of resting CT myocardial perfusion in patients with possible acute coronary syndrome. AJR Am J Roentgenol 2013;200:W450–W457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Iwasaki K, Matsumoto T. Myocardial perfusion defect in patients with coronary artery disease demonstrated by 64-multidetector computed tomography at rest. Clin Cardiol 2011;34:454–460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Taylor CA, Fonte TA, Min JK. Computational fluid dynamics applied to cardiac computed tomography for noninvasive quantification of fractional flow reserve: scientific basis. J Am Coll Cardiol 2013;61:2233–2241. [DOI] [PubMed] [Google Scholar]
- 67. Koo B-K, Erglis A, Doh J-H, Daniels DV, Jegere S, Kim H-S, Dunning A, DeFrance T, Lansky A, Leipsic J, Min JK. Diagnosis of ischemia-causing coronary stenoses by noninvasive fractional flow reserve computed from coronary computed tomographic angiograms. Results from the prospective multicenter DISCOVER-FLOW (Diagnosis of Ischemia-Causing Stenoses Obtained Via Noninvasive Fractional Flow Reserve) study. J Am Coll Cardiol 2011;58:1989–1997. [DOI] [PubMed] [Google Scholar]
- 68. Min JK, Leipsic J, Pencina MJ, Berman DS, Koo B-K, van Mieghem C, Erglis A, Lin FY, Dunning AM, Apruzzese P, Budoff MJ, Cole JH, Jaffer FA, Leon MB, Malpeso J, Mancini GBJ, Park S-J, Schwartz RS, Shaw LJ, Mauri L. Diagnostic accuracy of fractional flow reserve from anatomic CT angiography. JAMA 2012;308:1237–1245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Nørgaard BL, Leipsic J, Gaur S, Seneviratne S, Ko BS, Ito H, Jensen JM, Mauri L, de Bruyne B, Bezerra H, Osawa K, Marwan M, Naber C, Erglis A, Park S-J, Christiansen EH, Kaltoft A, Lassen JF, Botker HE, Achenbach S, NXT Trial Study Group. Diagnostic performance of noninvasive fractional flow reserve derived from coronary computed tomography angiography in suspected coronary artery disease: the NXT trial (Analysis of Coronary Blood Flow Using CT Angiography: Next Steps). J Am Coll Cardiol 2014;63:1145–1155. [DOI] [PubMed] [Google Scholar]
- 70. Min JK, Koo B-K, Erglis A, Doh J-H, Daniels DV, Jegere S, Kim H-S, Dunning AM, DeFrance T, Lansky A, Leipsic J. Usefulness of noninvasive fractional flow reserve computed from coronary computed tomographic angiograms for intermediate stenoses confirmed by quantitative coronary angiography. Am J Cardiol 2012;110:971–976. [DOI] [PubMed] [Google Scholar]
- 71. Achenbach S, Moselewski F, Ropers D, Ferencik M, Hoffmann U, Macneill B, Pohle K, Baum U, Anders K, Jang I-K, Daniel WG, Brady TJ. Detection of calcified and noncalcified coronary atherosclerotic plaque by contrast-enhanced, submillimeter multidetector spiral computed tomography: a segment-based comparison with intravascular ultrasound. Circulation 2004;109:14–17. [DOI] [PubMed] [Google Scholar]
- 72. Voros S, Rinehart S, Qian Z, Joshi P, Vazquez G, Fischer C, Belur P, Hulten E, Villines TC. Coronary atherosclerosis imaging by coronary CT angiography: current status, correlation with intravascular interrogation and meta-analysis. JACC Cardiovasc Imaging 2011;4:537–548. [DOI] [PubMed] [Google Scholar]
- 73. Maurovich-Horvat P, Ferencik M, Voros S, Merkely B, Hoffmann U. Comprehensive plaque assessment by coronary CT angiography. Nat Rev Cardiol 2014;11:390–402. [DOI] [PubMed] [Google Scholar]
- 74. Papadopoulou S-L, Garcia-Garcia HM, Rossi A, Girasis C, Dharampal AS, Kitslaar PH, Krestin GP, de Feyter PJ. Reproducibility of computed tomography angiography data analysis using semiautomated plaque quantification software: implications for the design of longitudinal studies. Int J Cardiovasc Imaging 2013;29:1095–1104. [DOI] [PubMed] [Google Scholar]
- 75. Kashiwagi M, Tanaka A, Kitabata H, Tsujioka H, Kataiwa H, Komukai K, Tanimoto T, Takemoto K, Takarada S, Kubo T, Hirata K, Nakamura N, Mizukoshi M, Imanishi T, Akasaka T. Feasibility of noninvasive assessment of thin-cap fibroatheroma by multidetector computed tomography. JACC Cardiovasc Imaging 2009;2:1412–1419. [DOI] [PubMed] [Google Scholar]
- 76. Leber AW, Becker A, Knez A, Ziegler von F, Sirol M, Nikolaou K, Ohnesorge B, Fayad ZA, Becker CR, Reiser M, Steinbeck G, Boekstegers P. Accuracy of 64-slice computed tomography to classify and quantify plaque volumes in the proximal coronary system: a comparative study using intravascular ultrasound. J Am Coll Cardiol 2006;47:672–677. [DOI] [PubMed] [Google Scholar]
- 77. Otsuka M, Bruining N, Van Pelt NC, Mollet NR, Ligthart JMR, Vourvouri E, Hamers R, de Jaegere P, Wijns W, Van Domburg RT, Stone GW, Veldhof S, Verheye S, Dudek D, Serruys PW, Krestin GP, de Feyter PJ. Quantification of coronary plaque by 64-slice computed tomography: a comparison with quantitative intracoronary ultrasound. Invest Radiol 2008;43:314–321. [DOI] [PubMed] [Google Scholar]
- 78. Marwan M, Taher MA, Meniawy ElK, Awadallah H, Pflederer T, Schuhbäck A, Ropers D, Daniel WG, Achenbach S. In vivo CT detection of lipid-rich coronary artery atherosclerotic plaques using quantitative histogram analysis: a head to head comparison with IVUS. Atherosclerosis 2011;215:110–115. [DOI] [PubMed] [Google Scholar]
- 79. Pundziute G, Schuijf JD, Jukema JW, Decramer I, Sarno G, Vanhoenacker PK, Reiber JHC, Schalij MJ, Wijns W, Bax JJ. Head-to-head comparison of coronary plaque evaluation between multislice computed tomography and intravascular ultrasound radiofrequency data analysis. J Am Coll Cardiol Interv 2008;1:176–182. [DOI] [PubMed] [Google Scholar]
- 80. Gauss S, Achenbach S, Pflederer T, Schuhbäck A, Daniel WG, Marwan M. Assessment of coronary artery remodelling by dual-source CT: a head-to-head comparison with intravascular ultrasound. Heart 2011;97:991–997. [DOI] [PubMed] [Google Scholar]
- 81. Maurovich-Horvat P, Schlett CL, Alkadhi H, Nakano M, Otsuka F, Stolzmann P, Scheffel H, Ferencik M, Kriegel MF, Seifarth H, Virmani R, Hoffmann U. The napkin-ring sign indicates advanced atherosclerotic lesions in coronary CT angiography. JACC Cardiovasc Imaging 2012;5:1243–1252. [DOI] [PubMed] [Google Scholar]
- 82. Hoffmann U, Moselewski F, Nieman K, Jang I-K, Ferencik M, Rahman AM, Cury RC, Abbara S, Joneidi-Jafari H, Achenbach S, Brady TJ. Noninvasive assessment of plaque morphology and composition in culprit and stable lesions in acute coronary syndrome and stable lesions in stable angina by multidetector computed tomography. J Am Coll Cardiol 2006;47:1655–1662. [DOI] [PubMed] [Google Scholar]
- 83. Pflederer T, Marwan M, Schepis T, Ropers D, Seltmann M, Muschiol G, Daniel WG, Achenbach S. Characterization of culprit lesions in acute coronary syndromes using coronary dual-source CT angiography. Atherosclerosis 2010;211:437–444. [DOI] [PubMed] [Google Scholar]
- 84. Kim SY, Kim K-S, Seung MJ, Chung JW, Kim JH, Mun SH, Lee YS, Lee JB, Ryu JK, Choi JY, Chang SG. The culprit lesion score on multi-detector computed tomography can detect vulnerable coronary artery plaque. Int J Cardiovasc Imaging 2010;26:245–252. [DOI] [PubMed] [Google Scholar]
- 85. Motoyama S, Kondo T, Sarai M, Sugiura A, Harigaya H, Sato T, Inoue K, Okumura M, Ishii J, Anno H, Virmani R, Ozaki Y, Hishida H, Narula J. Multislice computed tomographic characteristics of coronary lesions in acute coronary syndromes. J Am Coll Cardiol 2007;50:319–326. [DOI] [PubMed] [Google Scholar]
- 86. Kitagawa T, Yamamoto H, Horiguchi J, Ohhashi N, Tadehara F, Shokawa T, Dohi Y, Kunita E, Utsunomiya H, Kohno N, Kihara Y. Characterization of noncalcified coronary plaques and identification of culprit lesions in patients with acute coronary syndrome by 64-slice computed tomography. JACC Cardiovasc Imaging 2009;2:153–160. [DOI] [PubMed] [Google Scholar]
- 87. Yamamoto H, Kitagawa T, Ohashi N, Utsunomiya H, Kunita E, Oka T, Urabe Y, Tsushima H, Awai K, Kihara Y. Noncalcified atherosclerotic lesions with vulnerable characteristics detected by coronary CT angiography and future coronary events. J Cardiovasc Comput Tomogr 2013;7:192–199. [DOI] [PubMed] [Google Scholar]
- 88. Park H-B, Heo R, ó Hartaigh B, Cho I, Gransar H, Nakazato R, Leipsic J, Mancini GBJ, Koo B-K, Otake H, Budoff MJ, Berman DS, Erglis A, Chang H-J, Min JK. Atherosclerotic plaque characteristics by CT angiography identify coronary lesions that cause ischemia: a direct comparison to fractional flow reserve. JACC Cardiovasc Imaging 2015;8:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Ferencik M, Schlett CL, Ghoshhajra BB, Kriegel MF, Joshi SB, Maurovich-Horvat P, Rogers IS, Banerji D, Bamberg F, Truong QA, Brady TJ, Nagurney JT, Hoffmann U. A computed tomography-based coronary lesion score to predict acute coronary syndrome among patients with acute chest pain and significant coronary stenosis on coronary computed tomographic angiogram. Am J Cardiol 2012;110:183–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Puchner SB, Liu T, Mayrhofer T, Truong QA, Lee H, Fleg JL, Nagurney JT, Udelson JE, Hoffmann U, Ferencik M. High-risk plaque detected on coronary CT angiography predicts acute coronary syndromes independent of significant stenosis in acute chest pain: results from the ROMICAT-II trial. J Am Coll Cardiol 2014;64:684–692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Ferencik M, Liu T, Mayrhofer T, Puchner SB, Lu MT, Maurovich-Horvat P, Pope JH, Truong QA, Udelson JE, Peacock WF, White CS, Woodard PK, Fleg JL, Nagurney JT, Januzzi JL, Hoffmann U. Highly sensitive troponin I followed by advanced coronary artery disease assessment using computed tomography angiography improves acute coronary syndrome risk stratification accuracy and work-up in acute chest pain patients: results from ROMICAT II trial. JACC Cardiovasc Imaging 2015;8:1272–1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Schlett CL, Banerji D, Siegel E, Bamberg F, Lehman SJ, Ferencik M, Brady TJ, Nagurney JT, Hoffmann U, Truong QA. Prognostic value of CT angiography for major adverse cardiac events in patients with acute chest pain from the emergency department: 2-year outcomes of the ROMICAT trial. JACC Cardiovasc Imaging 2011;4:481–491. [DOI] [PMC free article] [PubMed] [Google Scholar]