Abstract
Aims
Blood pressure (BP) responses during dobutamine stress echocardiography (DSE) have not been systematically studied. Consequently, it is not known what constitutes a normal or an abnormal BP response to dobutamine stress. We sought to define the typical BP response during DSE of patients not known to have cardiovascular disease.
Methods and results
Of 24 134 patients who underwent DSE from November 2003 to December 2012 at Mayo Clinic, Rochester, MN, 2968 were selected for inclusion in this retrospective study. Excluded were patients with a history of hypertension, diabetes, or coronary artery disease, and those taking vasoactive medications. Patients who had baseline and/or stress-induced wall motion abnormalities were also excluded. The distribution of the study population's BP responses during DSE was Gaussian; we defined cut-point values for normative BP responses at 2 SD for each decade of age and for the whole study population. During DSE, systolic BP (SBP) increased from baseline to peak stress (Δ +2.9 ± 24 mmHg, P < 0.0001) and diastolic BP (DBP) decreased (Δ −7.4 ± 14 mmHg). BP changes were age and sex dependent; men and younger patients had greater ΔSBP and lesser ΔDBP, compared with women and older patients. Patients who received atropine had higher peak BP values than patients who did not receive atropine, due to greater ΔSBP (+7.4 ± 26 vs. −0.5 ± 22 mmHg, P < 0.0001) and lesser ΔDBP (−4 ± 14 vs. −9.7 ± 12 mmHg, P < 0.0001). This atropine effect was present in men and women, and was more pronounced in younger patients. The normative peak SBP values ranged from 82 to 182 mmHg.
Conclusion
BP responses during DSE vary and depend on patients' age, gender, and the use of atropine. We describe the typical BP responses seen during DSE and report normative reference values, which can be used for defining normal and abnormal BP responses to dobutamine stress.
Keywords: dobutamine stress echocardiography, blood pressure, atropine
Introduction
Dobutamine stress echocardiography (DSE) is a well-established diagnostic and prognostic stress imaging test, used for the evaluation of patients with suspected or established coronary artery disease.1–4 Although dobutamine is generally well tolerated and safe,4–10 abnormal blood pressure (BP) responses can occur and may lead to early discontinuation of the test.1,2 Even though DSE has been performed for longer than 25 years at many institutions, patterns of BP responses during DSE have not been systematically studied. In one study, published 20 years ago, dobutamine/atropine stress echocardiography was performed on 14 young healthy adult subjects.11 In prior studies, investigators used different BP cut-points to describe an abnormal BP response during DSE.8,12–16 These cut-points varied and were arbitrarily established or extrapolated from exercise stress data. Additionally, patients in those studies had hypertension, diabetes, or established coronary disease. These conditions and their treatments can have an effect on BP responses during DSE and on the stress echocardiographic findings. There are few published studies examining the effect of atropine administration on BP responses during DSE.17,18 Normative reference values for BP responses during DSE are needed to reach agreement on what the definition(s) of abnormal BP responses to dobutamine stress should be.
We sought to define the typical BP response to DSE in a large contemporary series of patients not known to have cardiovascular disease who had normal findings on DSE. We also evaluated the impact of the addition of atropine on BP responses during DSE. Finally, we propose cut-point values for normative systolic blood pressure (SBP) and diastolic blood pressure (DBP) responses to be used as reference values for future studies.
Methods
Study population
All patients (n = 24 134) who underwent DSE at Mayo Clinic, Rochester, MN, from November 2003 to December 2012 were candidates for this retrospective study. Patients' clinical characteristics were abstracted from their medical records by specially trained registered nurses at the time of their stress echocardiograms. These data, along with each patient's baseline and stress clinical and echocardiographic data, were entered into an institutional stress echocardiography research database. Of the patients, 1223 patients did not give permission to use their medical records for research purposes. We excluded patients who had a history of hypertension (n = 16 488), diabetes mellitus (n = 6619), coronary artery disease (defined as a history of myocardial infarction, prior percutaneous coronary revascularization or coronary artery bypass graft surgery) (n = 5810), or at least moderate valvular heart disease (n = 952). Patients taking β-blockers, calcium-blockers, or inhibitors of the renin–angiotensin–aldosterone system were excluded, as were patients with baseline and/or stress-induced echocardiographic regional wall motion abnormalities (n = 6834). After exclusions, the study population comprised 2968 patients.
DSE protocol
After a 3-h fast, dobutamine was administered intravenously by an infusion pump at a starting dose of 5 µg/kg/min. After 3 min, the dose was increased to 10 µg/kg/min and then by 10 µg/kg/min every 3 min, up to a maximal dose of 40 µg/kg/min if needed. Intravenous atropine was administered to patients who did not achieve their target heart rate (HR), which was 85% of age-predicted maximal HR,1 calculated by subtracting the age of the patient from 220. Atropine was administrated in 0.25 mg dose increments every 1 min, up to a maximum of 2 mg if necessary.18–20 Atropine was given at the end of the 20 µg/kg/min stage if the HR was <90 bpm, at the end of the 30 µg/kg/min stage if <70% of age-predicted maximal HR, and at the end of the 40 µg/kg/min stage if <85% of age-predicted maximal HR. The criteria for terminating the test were those recommended in the guidelines1,2 and included severe hypertension (SBP > 240 or DBP > 120 mmHg) and hypotension (SBP < 90 mmHg if associated with symptoms).
BP measurements
Systolic and diastolic BP measurements were made by specially trained registered nurses using a sphygmomanometer with patients in the left lateral decubitus position: at baseline and every 3 min during the test, near or at peak stress and in recovery. ΔSBP was defined as SBP at peak stress minus SBP at baseline. ΔDBP was defined as DBP at peak stress minus DBP at baseline. Peak SBP and peak DBP were defined as SBP and DBP at peak stress.
Echocardiographic analysis
Two-dimensional digitized and videotaped echocardiographic images were acquired at baseline, during the stress test, and in recovery, according to a previously published protocol.21 The left ventricular ejection fraction (EF) was determined by either visual assessment or a modification of the Quinones method.22 Wall motion was assessed with a 16-segment model proposed by the American Society of Echocardiography.23
Statistical analysis
Clinical and echocardiographic data were summarized using means and standard deviations (SDs) for continuous variables and percentages for dichotomous or qualitative variables. Data are presented according to sex, age, and usage of atropine in addition to dobutamine. BP values at every stage of dobutamine infusion were also summarized. T-tests, χ2 tests, and analysis of variance (ANOVA) were used when appropriate. Paired sample t-test and ANOVA for paired data were used to compare intragroup differences. A significance level of 0.05 was considered statistically significant. Cut-point values were defined based on the distribution of peak systolic and diastolic BP values, which was normal (Gaussian) for both variables. We added 2 SDs for the upper limit and subtracted 2 SDs for the lower limit. A logistic regression analysis was used to identify predictors of hypertensive and hypotensive SBP responses during DSE. The variables considered were age, sex, baseline SBP, atropine usage, EF at baseline, and EF at peak stress.
Results
Of the 2968 patients, 1335 (45%) underwent DSE for pre-operative assessment, 1283 (43%) for assessment of chest pain or dyspnoea, and 350 (12%) for coronary artery disease screening or abnormal electrocardiography. The mean age of the study population was 62 ± 13 years, and 1658 (56%) were women. The ethnicity of the study population, by self-reporting, was as follows: 2644 were white, 48 black, 27 Asian, 11 native-American, and 219 other or not known.
BP response
During dobutamine infusion, the SBP response was ‘flat’ (<10 mmHg increase or decrease compared with baseline) in 1118 patients (38%). The SBP increased by 10 mmHg or more in 1064 patients (36%), and decreased by 10 mmHg or more in 786 patients (26%). Overall, the SBP increased slightly from baseline to peak stress, the DBP and mean BP decreased, and the pulse pressure increased (Table 1).
Table 1.
Blood pressure, heart rate, rate-pressure product, and ejection fraction responses during DSE
| Baseline | Peak stress | Delta | |
|---|---|---|---|
| SBP (mmHg) | 129 ± 19 | 132 ± 25 | +2.9 ± 24 |
| DBP (mmHg) | 76 ± 12 | 68 ± 14 | −7.4 ± 14 |
| Mean BP (mmHg) | 94 ± 13 | 90 ± 16 | −4 ± 15 |
| Pulse pressure (mmHg) | 53 ± 14 | 64 ± 18 | +9.3 ± 19 |
| Heart rate (bpm) | 72 ± 12 | 138 ± 12 | 66 ± 16 |
| Rate-pressure product | 9256 ± 2068 | 18 211 ± 3876 | 8952 ± 4100 |
| EF (%) | 62 ± 5 | 75 ± 4 | 13 ± 4 |
All P-values at baseline vs. peak stress: <0.0001.
DBP, diastolic blood pressure; EF, ejection fraction; SBP, systolic blood pressure.
Compared with men, women had less change in SBP during DSE (ΔSBP +1.1 ± 23 for women vs. +5 ± 24 mmHg for men, P < 0.0001) and greater change in DBP (ΔDBP −8 ± 13 for women vs. −6.7 ± 13 mmHg for men, P = 0.016).
The degree of BP change during dobutamine infusion was age related (Table 2). The ΔSBP of younger patients was greater than that of older patients, with a downward trend across decades and negative values in patients 70 years and older. Younger patients had a lesser decrease in DBP values than older patients.
Table 2.
Blood pressure and heart rate responses during DSE, by age groups
| Age (years) | <50 | 50–59 | 60–69 | ≥70 | P-value |
|---|---|---|---|---|---|
| No. of patients | 478 | 714 | 873 | 903 | |
| Baseline SBP (mmHg) | 122 ± 17 | 123 ± 17 | 129 ± 19 | 138 ± 19 | <0.0001 |
| Peak SBP (mmHg) | 137 ± 25 | 130 ± 25 | 131 ± 25 | 133 ± 23 | <0.0001 |
| Delta SBP (mmHg) | +16 ± 23 | +6 ± 22 | +1 ± 23 | −6 ± 23 | <0.0001 |
| Baseline DBP (mmHg) | 76 ± 12 | 75 ± 12 | 76 ± 12 | 76 ± 11 | ns |
| Peak DBP (mmHg) | 74 ± 15 | 70 ± 14 | 67 ± 14 | 66 ± 12 | <0.0001 |
| Delta DBP (mmHg) | −2 ± 15 | −6 ± 13 | −9 ± 13 | −10 ± 12 | <0.0001 |
| Baseline PP (mmHg) | 46 ± 11 | 49 ± 12 | 53 ± 13 | 62 ± 15 | <0.0001 |
| Peak PP (mmHg) | 64 ± 17 | 60 ± 18 | 64 ± 19 | 66 ± 18 | <0.0001 |
| Delta PP (mmHg) | +18 ± 17 | +12 ± 17 | +10 ± 18 | +4 ± 19 | <0.0001 |
| Baseline MBP (mmHg) | 91 ± 13 | 91 ± 13 | 94 ± 13 | 97 ± 12 | <0.0001 |
| Peak MBP (mmHg) | 95 ± 17 | 90 ± 16 | 88 ± 16 | 88 ± 14 | <0.0001 |
| Delta MBP (mmHg) | +4 ± 16 | −2 ± 14 | −5 ± 15 | −9 ± 14 | <0.0001 |
| Baseline HR (bpm) | 73 ± 13 | 73 ± 12 | 71 ± 12 | 70 ± 11 | <0.0001 |
| Peak HR (bpm) | 151 ± 11 | 142 ± 9 | 135 ± 9 | 129 ± 10 | <0.0001 |
| Baseline RPP | 8848 ± 2153 | 8960 ± 1946 | 9248 ± 2101 | 9714 ± 1997 | <0.0001 |
| Peak RPP | 20 759 ± 4269 | 18 524 ± 3923 | 17 638 ± 3460 | 17 170 ± 3333 | <0.0001 |
| Delta RPP | 11 909 ± 4403 | 9555 ± 3996 | 8387 ± 3617 | 7460 ± 3516 | <0.0001 |
DBP, diastolic blood pressure; HR, heart rate; MBP, mean blood pressure; ns, non-significant; PP, pulse pressure; RPP, rate-pressure product; SBP, systolic blood pressure.
BP response and atropine administration
A total of 1264 (43%) patients received atropine. Men received atropine more frequently than women (51 vs. 37%, P < 0.0001). Patients who received atropine had significantly higher peak HR and BP values, compared with patients who did not receive atropine. ΔHR was higher in patients who received atropine (73 ± 15 bpm), compared with patients who did not (61 ± 15, P < 0.0001). The ΔSBP values were +7.4 ± 26 for patients who received atropine and −0.5 ± 22 mmHg (P < 0.0001) for patients who did not; the corresponding ΔDBP values were −4.4 ± 14 and −9.7 ± 12 mmHg, respectively (P < 0.0001). This effect of atropine was present in men (ΔSBP and ΔDBP +8.6 ± 26 and −4.4 ± 15 with atropine vs. +1.7 ± 23 and −9 ± 12 without atropine) and in women (+6.3 ± 25 and −4.3 ± 14 with atropine vs. −1.9 ± 21 and −10 ± 12 without atropine). Atropine was administrated to 64% of patients <50 years, to 55% of patients between 50 and 59 years, to 39% of patients between 60 and 69 years, and to 25% of patients ≥70 years. There were greater SBP changes in younger patients who received atropine and lesser DBP changes in all age groups who received atropine (Figure 1).
Figure 1.
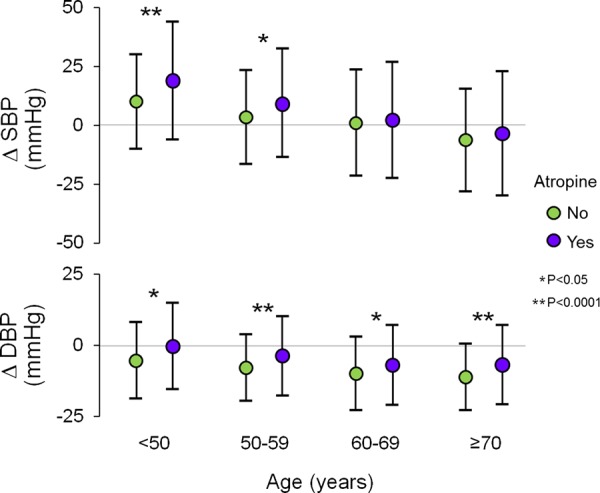
Blood pressure response during DSE, by age and the use of atropine. ΔSBP was higher and ΔDBP was lower in patients who received atropine, compared with patients who did not. BP values were higher in younger patients. Data presented as mean value ± 1 SD. ΔDBP, change in diastolic blood pressure; ΔSBP, change in systolic blood pressure.
HR response, symptoms, and ECG results
A total of 2196 patients (74%) achieved target HR; 563 patients (19%) and 227 patients (7%) achieved 80 to 84.9% and <80% of age-predicted maximal HR, respectively. The patients who did not achieve target HR were younger (57 ± 12 vs. 64 ± 13 years, P < 0.0001). Although the peak SBP achieved was lower in patients who did not achieve target HR (128 ± 27 vs. 134 ± 23 mmHg, P < 0.0001), the upper ranges (2 SDs) for the two subgroups were comparable (182 vs. 180 mmHg). Similarly, although the peak DBP was lower (67 ± 15 vs. 69 ± 13 mmHg, P < 0.0015) in patients who did not achieve target HR, the upper ranges (2 SDs) for the two subgroups were comparable (97 vs. 95 mmHg).
During DSE, chest pain developed in 12% of patients, dyspnoea in 8%, headache in 11%, nausea in 9%, and shivering or anxiety in 19%. Overall, 93% of patients had stress ECG results that were negative for ischaemia, 2% were positive for ischaemia, and 5% were uninterpretable. Complications were rare; 19 patients developed sustained supraventricular tachycardia. All of these patients went back into sinus rhythm after administration of intravenous metoprolol or esmolol. Another 16 patients developed atrial fibrillation. Eight of these patients went back into sinus rhythm during extended recovery period monitoring after administration of an intravenous β-blocker. The other eight patients had HR slowing; at follow-up within 24 h, sinus rhythm had returned spontaneously in seven patients and one patient underwent direct current cardioversion. There were no cases of sustained ventricular tachycardia, ventricular fibrillation, or myocardial infarction.
Patients receiving dobutamine dose of 40 µg/kg/min
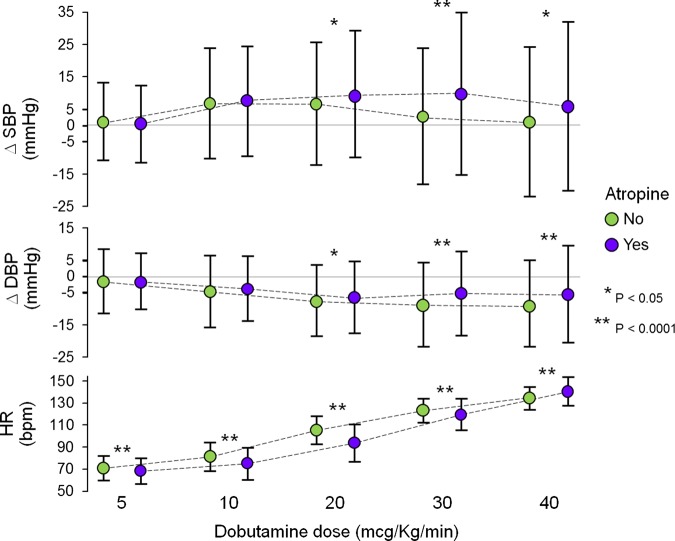
A total of 1449 patients received the full dobutamine dose of 40 µg/kg/min. Of these patients, 855 (59%) also received atropine. There was a positive ΔSBP from baseline to peak dose (+4 ± 25 mmHg, P < 0.0001) and negative ΔDBP (−7 ± 14 mmHg, P < 0.0001), resulting in slightly negative mean BP (−3 ± 16 mmHg, P < 0.0001). ΔSBP was significantly greater in men than in women (+6 ± 25 vs. +2 ± 25 mmHg, P= 0.016), and in younger patients than in older patients. There was a significant increase in ΔSBP at a dobutamine dose of 10 µg/kg/min. The ΔSBP increase persisted at higher dobutamine doses in patients who received atropine, but not in patients who did not receive atropine (Figure 2). There was a progressive downward trend in ΔDBP in both groups, but the extent of reduction at each dobutamine stage was lower in patients who received atropine, compared with patients who did not. The HR increased progressively in both groups. The rise was significantly slower in patients who received atropine (Figure 2).
Figure 2.
Blood pressure and HR responses in patients who received a dobutamine dose of 40 µg/kg/min, by the use of atropine. Changes from baseline, during each dobutamine stage. Data presented as mean value ± 1 SD. ΔDBP, change in diastolic blood pressure; ΔSBP, change in systolic blood pressure; HR, heart rate.
Patients who received atropine developed symptoms more frequently than patients who did not receive atropine, including chest pain (17 vs. 8%, P < 0.0001), headache (13 vs. 9%, P = 0.03), nausea (15 vs. 8%, P = 0.0003), and shivering or anxiety (25 vs. 17%, P = 0.0003). The frequency of dyspnoea was the same in both groups (9%).
Normative BP responses during DSE
Based on the Gaussian distribution of the BP response to DSE, cut-point values for normative BP response to DSE were generated, both for the whole study population and for each decade of age (Table 3). To facilitate the utilization of these cut-point values in clinical practice, we propose using two unique upper values (one systolic and one diastolic) and two unique lower values (one systolic and one diastolic), obtained from the overall study population. The proposed normative SBP value at peak stress ranges from 82 to 182 mmHg. The proposed normative DBP value at peak stress ranges from 40 to 96 mmHg.
Table 3.
Cut-points for normative blood pressure response during DSE, based on peak SBP and peak DBP means ±2 SDs, overall and by age
| Age (years) | <50 | 50–59 | 60–69 | ≥70 | Overall |
|---|---|---|---|---|---|
| Peak SBP (mmHg) | 137 ± 25 | 130 ± 25 | 131 ± 25 | 133 ± 23 | 132 ± 25 |
| +2 SD | 187 | 180 | 181 | 179 | 182 |
| −2 SD | 87 | 80 | 81 | 87 | 82 |
| Peak DBP (mmHg) | 74 ± 15 | 70 ± 14 | 67 ± 14 | 66 ± 12 | 68 ± 14 |
| +2 SD | 104 | 98 | 95 | 90 | 96 |
| −2 SD | 44 | 42 | 39 | 42 | 40 |
DBP, diastolic blood pressure; SBP, systolic blood pressure; SD, standard deviation.
Predictors of abnormal BP response
As defined by the peak SBP, 66 patients had a hypertensive response and 51 had a hypotensive response. Multivariate predictors of a hypertensive response were male sex [odds ratio (OR) 2.6, confidence interval (CI) 1.5–4.5, P = 0.003], age (OR 0.7 per 10-year increase, CI 0.5–0.8, P < 0.001), atropine usage (OR 2.6, CI 1.5–4.6, P = 0.0005), and baseline SBP (OR 1.6 per 10 mmHg increase, CI 1.4–1.9, P < 0.0001). The only independent predictor of a hypotensive response was baseline SBP (OR 0.5 per 10 mmHg increase, CI 0.4–0.6, P < 0.0001). Left ventricular EF at peak stress was not a multivariate predictor of a hypotensive response.
Discussion
The typical BP response during DSE in a population without known cardiovascular disease and not on any vasoactive medication consists of a slight increase in SBP and a decrease in DBP. BP response is greater in men than in women, and in younger than in older patients, and is most pronounced in patients who receive atropine, regardless of sex and age. On the basis of these results, we propose novel normative values for BP response to dobutamine stress.
BP and HR response
Dobutamine is a sympathomimetic agent that increases myocardial oxygen consumption through predominantly inotropic and chronotropic effects (β-1-adrenergic cardiac receptor agonism).6,24,25 It also has weaker peripheral actions, mostly as a vasodilator (β-2-adrenergic receptor agonism) promoting a slight baroreflex-mediated further increase in HR, and minimally as a vasopressor (α-1-adrenergic receptor agonism).6,14,24 The net effect on BP is the result of increased myocardial contractility combined with the sum of opposing peripheral vascular effects. In our study population, a predominant dobutamine-induced vasodilating effect was likely the main mechanism for the slight decrease in DBP we observed. This effect is likely counterbalanced in systole by a dobutamine-induced increase in cardiac output, resulting in a slight increase in SBP. In addition, we observed a significant increase in HR and rate-pressure product. Similarly, in prior animal model studies, rate-pressure product increased but mean aortic pressure did not change significantly with dobutamine infusion.26,27 Healthy young men who received both dobutamine and atropine prior to positron emission tomography had an increase in SBP, DBP, and rate-pressure product.28 Prior clinical studies described an overall increase in DBP during DSE13,15 and an increase in SBP that was greater than that of our study.3,4,13,15,16 These studies included patients with cardiovascular co-morbidities who were on vasoactive medications, factors that can influence the cardiovascular response to dobutamine stress. For example, patients who have hypertension often have elevated peripheral vascular resistance;29 an increase in cardiac output by dobutamine infusion may not be offset by prompt peripheral vasodilation in a subset of these patients. Additionally, the modest α-1-adrenergic vasoconstrictive effect of dobutamine may be exaggerated in hypertensive patients with sympathetic nervous system hypersensitivity. β-Adrenergic receptor antagonism with β-blocker therapy may result in an exaggerated BP response to dobutamine and atropine in susceptible patients; further studies are needed to address this hypothesis.
Sex differences
We found that women had lower peak SBP and DBP values during DSE than did men, whether atropine was administrated or not. These findings were consistent with those of a prior study.30 Women who did not receive atropine had negative values of ΔSBP, emphasizing the low likelihood of women developing high SBP in response to dobutamine. In contrast, other studies reported no sex differences in BP response or greater SBP increases during DSE in women than in men.3,31 However, in contrast to the present study, in those studies women had greater baseline SBP values than men or patients were taking multiple cardiovascular drugs. The precise mechanism underlying these sex differences in BP response during DSE is unclear, but arterial BP dissimilarities between men and women are recognized in the literature.32
Age differences
In the present study, the increase in SBP during DSE was greater in younger than in older patients and tended to decline with age, becoming a SBP decrease in patients ≥70 years of age. Conversely, the DBP decrease during DSE was greater with advancing age. As a result, older patients had lower BP in response to DSE, consistent with previous studies.9,25 A clear explanation underlying difference of BP responses between ages has not been defined and different mechanisms likely contribute. Age-related changes in adrenergic and baroreflex responsiveness have been demonstrated33–35 and include a diminished stress-induced HR increase in the elderly.34 This could influence at least SBP changes, by a lesser effect on cardiac output compared with younger patients. Furthermore, it is known that vascular compliance decreases with age, leading to a lower effectiveness in compensatory BP changes, along with sharp falls in DBP in the elderly.36 Further information regarding cardiac output and arterial stiffness would be needed to fully explain these BP responses.
Effect of atropine
Atropine use during DSE is safe and enhances the diagnostic accuracy of the test by contributing to an increase in HR and myocardial oxygen demand.18,19,37 Atropine, at the doses used during DSE, exerts its effect through a parasympatholytic effect (competitive antagonist of muscarinic acetylcholine receptors) at the cardiac level,37 countering the decrease in HR mediated by vagal tone. Studies on animals demonstrated that atropine has no direct effects on peripheral vasculature.38 The present study shows that patients who received atropine during DSE had higher peak BP values than patients who did not receive atropine, regardless of sex and age. This corresponded to a more pronounced ΔSBP from baseline to peak stress and a less pronounced negative ΔDBP. Peak BP values were especially higher in younger patients, who received atropine more often than older patients. The difference in ΔBP values between patients who received atropine and patients who did not were statistically significant at a dobutamine dose of 20 µg/kg/min, corresponding to the earliest time at which atropine was administered. Given the absence of atropine effect on the peripheral vasculature, its effects on BP would have to be due to its positive chronotropic effect. It is also possible that the higher frequency of symptoms that developed in patients receiving atropine contributed to the higher BP values. Few, but consistent hints of higher BP values in patients who received atropine, compared with patients who did not, have been reported in previous DSE studies.10,13
BP response cut-points
The expected or typical BP responses during DSE have not been systematically defined, and the cut-points used in previous studies to describe abnormal BP responses were arbitrarily established.8,12–16 On the basis of our results, we propose novel BP response cut-points for DSE, derived from a large population of patients without known cardiovascular disease and who were not on vasoactive medications. These data may help facilitate broad agreement regarding what the definition(s) of an abnormal BP response to dobutamine stress should be. Moreover, they constitute criteria that can be used for future studies regarding the clinical implications of abnormal BP responses during DSE.
Limitations
A limitation is the challenge of defining ‘normal’ BP responses during DSE in patients who are referred for testing. It is unlikely, however, that a study defining the expected or normal BP responses during DSE in healthy adult volunteers of all ages will ever be performed on a large scale. In the present study, it is possible that some patients had coexisting medical conditions that could have affected their BP responses to DSE even though all patients with known cardiovascular disease, those taking vasoactive medications, and those who had abnormal stress echocardiograms were excluded. A complete explanation of the mechanisms underlying BP pressure variations is beyond the scope of this study.
Conclusions
This is the first study to define typical BP responses during DSE in a large population of adult patients of all ages not known to have cardiovascular disease and not taking vasoactive medications. The existence of cut-off values for normal BP responses, rigorously established in this study, should aid in the recognition of abnormal BP responses during DSE. Moreover, these reference values should serve as a robust foundation for future studies, which could be aimed at answering questions that remain regarding the clinical implications of abnormal BP responses during DSE.
Conflict of interest: None declared.
References
- 1. Pellikka PA, Nagueh SF, Elhendy AA, Kuehl CA, Sawada SG. American Society of Echocardiography recommendations for performance, interpretation, and application of stress echocardiography. J Am Soc Echocardiogr 2007;20:1021–41. [DOI] [PubMed] [Google Scholar]
- 2. Sicari R, Nihoyannopoulos P, Evangelista A, Kasprzak J, Lancellotti P, Poldermans D et al. . Stress echocardiography expert consensus statement: European Association of Echocardiography (EAE) (a registered branch of the ESC). Eur J Echocardiogr 2008;9:415–37. [DOI] [PubMed] [Google Scholar]
- 3. Biagini E, Elhendy A, Bax JJ, Rizzello V, Schinkel AF, van Domburg RT et al. . Seven-year follow-up after dobutamine stress echocardiography: impact of gender on prognosis. J Am Coll Cardiol 2005;45:93–7. [DOI] [PubMed] [Google Scholar]
- 4. Sawada SG, Segar DS, Ryan T, Brown SE, Dohan AM, Williams R et al. . Echocardiographic detection of coronary artery disease during dobutamine infusion. Circulation 1991;83:1605–14. [DOI] [PubMed] [Google Scholar]
- 5. Varga A, Garcia MA, Picano E. Safety of stress echocardiography (from the International Stress Echo Complication Registry). Am J Cardiol 2006;98:541–3. [DOI] [PubMed] [Google Scholar]
- 6. Mertes H, Sawada SG, Ryan T, Segar DS, Kovacs R, Foltz J et al. . Symptoms, adverse effects, and complications associated with dobutamine stress echocardiography. Experience in 1118 patients. Circulation 1993;88:15–9. [DOI] [PubMed] [Google Scholar]
- 7. Secknus MA, Marwick TH. Evolution of dobutamine echocardiography protocols and indications: safety and side effects in 3,011 studies over 5 years. J Am Coll Cardiol 1997;29:1234–40. [DOI] [PubMed] [Google Scholar]
- 8. Mathias W Jr, Arruda A, Santos FC, Arruda AL, Mattos E, Osorio A et al. . Safety of dobutamine-atropine stress echocardiography: a prospective experience of 4,033 consecutive studies. J Am Soc Echocardiogr 1999;12:785–91. [DOI] [PubMed] [Google Scholar]
- 9. Chenzbraun A, Khoury Z, Gottlieb S, Keren A. Impact of age on the safety and the hemodynamic response pattern during high dose dobutamine echocardiography. Echocardiography 1999;16:135–42. [DOI] [PubMed] [Google Scholar]
- 10. Poldermans D, Fioretti PM, Boersma E, Thomson IR, Cornel JH, ten Cate FJ et al. . Dobutamine-atropine stress echocardiography in elderly patients unable to perform an exercise test. Hemodynamic characteristics, safety, and prognostic value. Arch Intern Med 1994;154:2681–6. [DOI] [PubMed] [Google Scholar]
- 11. Nixdorff U, Wagner S, Erbel R, Weitzel P, Mohr-Kahaly S, Meyer J. Normalwerte fur die dobutamin-stressechokardiographie. Dtsch Med Wochensc 1995;120:1761–7. [DOI] [PubMed] [Google Scholar]
- 12. Rosamond TL, Vacek JL, Hurwitz A, Rowland AJ, Beauchamp GD, Crouse LJ. Hypotension during dobutamine stress echocardiography: initial description and clinical relevance. Am Heart J 1992;123:403–7. [DOI] [PubMed] [Google Scholar]
- 13. Lee CY, Pellikka PA, Shub C, Sinak LJ, Seward JB. Hypertensive response during dobutamine stress echocardiography. Am J Cardiol 1997;80:970–1. [DOI] [PubMed] [Google Scholar]
- 14. Sorrentino MJ, Marcus RH, Lang RM. Left ventricular outflow tract obstruction as a cause for hypotension and symptoms during dobutamine stress echocardiography. Clin Cardiol 1996;19:225–30. [DOI] [PubMed] [Google Scholar]
- 15. Cortigiani L, Zanetti L, Bigi R, Desideri A, Fiorentini C, Nannini E. Safety and feasibility of dobutamine and dipyridamole stress echocardiography in hypertensive patients. J Hypertens 2002;20:1423–9. [DOI] [PubMed] [Google Scholar]
- 16. Marcovitz PA, Bach DS, Mathias W, Shayna V, Armstrong WF. Paradoxic hypotension during dobutamine stress echocardiography: clinical and diagnostic implications. J Am Coll Cardiol 1993;21:1080–6. [DOI] [PubMed] [Google Scholar]
- 17. Mazeika PK, Nadazdin A, Oakley CM. Clinical significance of abrupt vasodepression during dobutamine stress echocardiography. Am J Cardiol 1992;69:1484–6. [DOI] [PubMed] [Google Scholar]
- 18. Ling LH, Pellikka PA, Mahoney DW, Oh JK, McCully RB, Roger VL et al. . Atropine augmentation in dobutamine stress echocardiography: role and incremental value in a clinical practice setting. J Am Coll Cardiol 1996;28:551–7. [DOI] [PubMed] [Google Scholar]
- 19. Fioretti PM, Poldermans D, Salustri A, Forster T, Bellotti P, Boersma E et al. . Atropine increases the accuracy of dobutamine stress echocardiography in patients taking beta-blockers. Eur Heart J 1994;15:355–60. [DOI] [PubMed] [Google Scholar]
- 20. Lessick J, Mutlak D, Rinkevich D, Markiewicz W, Reisner SA. Prospective study of early atropine use in dobutamine stress echocardiography. Eur J Echocardiogr 2000;1:257–62. [DOI] [PubMed] [Google Scholar]
- 21. Attenhofer CH, Pellikka PA, Oh JK, Roger VL, McCully RB, Shub C et al. . Is review of videotape necessary after review of digitized cine-loop images in stress echocardiography? A prospective study in 306 patients. J Am Soc Echocardiogr 1997;10:179–84. [DOI] [PubMed] [Google Scholar]
- 22. Quinones MA, Waggoner AD, Reduto LA, Nelson JG, Young JB, Winters WL Jr et al. . A new, simplified and accurate method for determining ejection fraction with two-dimensional echocardiography. Circulation 1981;64:744–53. [DOI] [PubMed] [Google Scholar]
- 23. Lang RM, Badano LP, Mor-Avi V, Afilalo J, Armstrong A, Ernande L et al. . Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur Heart J Cardiovasc Img 2015;16:233–71. [DOI] [PubMed] [Google Scholar]
- 24. Ruffolo RR Jr, Spradlin TA, Pollock GD, Waddell JE, Murphy PJ. Alpha and beta adrenergic effects of the stereoisomers of dobutamine. J Pharmacol Exp Ther 1981;219:447–52. [PubMed] [Google Scholar]
- 25. Poldermans D, Boersma E, Fioretti PM, van Urk H, Boomsma F, Man in 't Veld AJ. Cardiac chronotropic responsiveness to beta-adrenoceptor stimulation is not reduced in the elderly. J Am Coll Cardiol 1995;25:995–9. [DOI] [PubMed] [Google Scholar]
- 26. Le E, Bin J-P, Coggins MP, Wei K, Lindner JR, Kaul S. Relation between myocardial oxygen consumption and myocardial blood volume: a study using myocardial contrast echocardiography. J Am Soc Echocardiogr 2001;15:857–63. [DOI] [PubMed] [Google Scholar]
- 27. Bin J-P, Le E, Jayaweera AR, Coggins MP, Wei K, Kaul S. Direct effects of dobutamine on the coronary microcirculation: comparison with adenosine using myocardial contrast echocardiography. J Am Soc Echocardiogr 2003;16:871–9. [DOI] [PubMed] [Google Scholar]
- 28. Tadamura E, Hidehiro I, Matsumoto K, Mamede M, Kubo S, Toyoda H et al. . Comparison of myocardial blood flow during dobutamine-atropine infusion with that after dipyridamole administration in normal men. J Am Coll Cardiol 2001;37:130–6. [DOI] [PubMed] [Google Scholar]
- 29. Vlachopoulos C, Aznaouridis K, Stefanadis C. Prediction of cardiovascular events and all-cause mortality with arterial stiffness: a systematic review and meta-analysis. J Am Coll Cardiol 2010;55:1318–27. [DOI] [PubMed] [Google Scholar]
- 30. Tsutsui JM, Falcao SN, Dourado PM, Lima MF, Alves AA, Guerra VC et al. . Gender differences in chronotropic and hemodynamic responses during dobutamine-atropine stress echocardiography. Echocardiography 2007;24:843–50. [DOI] [PubMed] [Google Scholar]
- 31. Elhendy A, Geleijnse ML, van Domburg RT, Nierop PR, Poldermans D, Bax JJ et al. . Gender differences in the accuracy of dobutamine stress echocardiography for the diagnosis of coronary artery disease. Am J Cardiol 1997;80:1414–8. [DOI] [PubMed] [Google Scholar]
- 32. Mancia G, Fagard R, Narkiewicz K, Redon J, Zanchetti A, Bohm M et al. . 2013 ESH/ESC Practice Guidelines for the Management of Arterial Hypertension. Blood Press 2014;23:3–16. [DOI] [PubMed] [Google Scholar]
- 33. Docherty JR. Cardiovascular responses in ageing: a review. Pharmacol Rev 1990;42:103–25. [PubMed] [Google Scholar]
- 34. Lakatta EG. Changes in cardiovascular function with aging. Eur Heart J 1990;11(Suppl C):22–9. [DOI] [PubMed] [Google Scholar]
- 35. Van Brummelen P, Buhler FR, Kiowski W, Amann FW. Age-related decrease in cardiac and peripheral vascular responsiveness to isoprenaline: studies in normal subjects. Clin Sci (Lond) 1981;60:571–7. [DOI] [PubMed] [Google Scholar]
- 36. Laurent S, Cockcroft J, Van Bortel L, Boutouyrie P, Giannattasio C, Hayoz D et al. . Expert consensus document on arterial stiffness: methodological issues and clinical applications. Eur Heart J 2006;27:2588–605. [DOI] [PubMed] [Google Scholar]
- 37. Kociolek LK, Bierig SM, Herrmann SC, Labovitz AJ. Efficacy of atropine as a chronotropic agent in heart transplant patients undergoing dobutamine stress echocardiography. Echocardiography 2006;23:383–7. [DOI] [PubMed] [Google Scholar]
- 38. Liu SQ, Zang WJ, Li ZL, Yu XJ, Li BP. Effect of atropine on denervated rabbit ear blood vessels. J Cardiovasc Pharmacol 2004;43:99–105. [DOI] [PubMed] [Google Scholar]