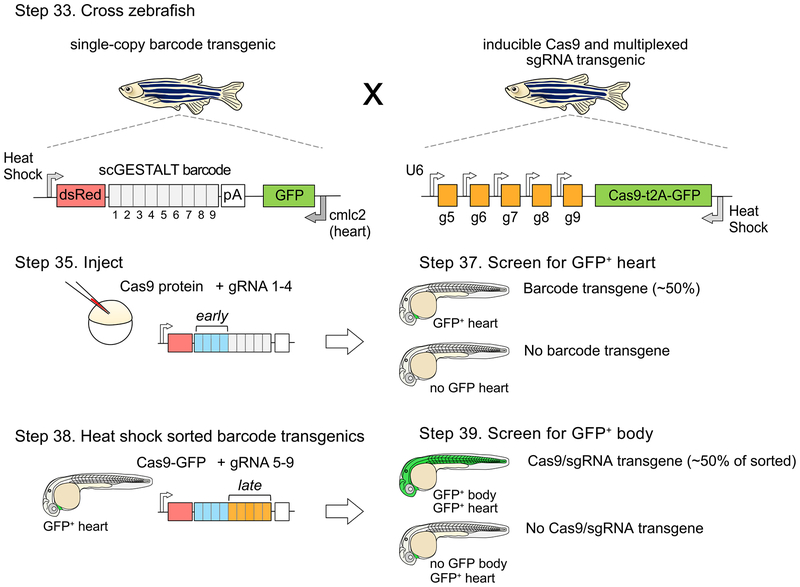
Figure 2. Strategy for a CRISPR-Cas9 system that enables early and late barcode editing.
Zebrafish with single-copy heat shock promoter-driven scGESTALT barcode (to promote ubiquitous barcode expression at stage of interest) are crossed to zebrafish that express heat shock-inducible Cas9 and U6-driven sgRNAs 5–9. The barcode is cloned downstream of the dsRed coding sequence and upstream of the SV40 polyadenylation sequence (pA). Resulting embryos are injected with Cas9 protein and sgRNAs 1–4 at the one-cell stage (blue bars; early editing). The embryos are screened for GFP positive heart transgenics (cmlc2 promoter drives heart-specific GFP expression) at 30 hpf to identify embryos containing the barcode transgene, and sorted embryos are heat shocked to induce transgenic Cas9 for a second round of editing (orange bars; late editing). The embryos are screened again for ubiquitous GFP expression (Cas9 is linked to GFP with a t2A self-cleaving peptide), which indicates successful Cas9 transgene induction. Double transgenic embryos are grown for downstream profiling, and heat shocked at time of interest (e.g. juvenile stage 23–25 dpf) to induce expression of the edited barcode array prior to scRNA-seq analysis. Protocol steps for each stage are indicated. Adapted with permission from ref72.