Figure 1.
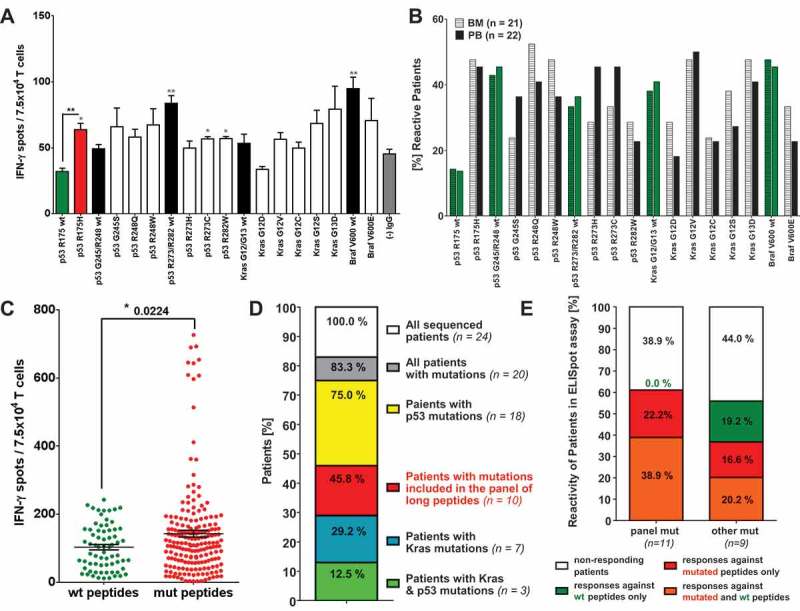
Relevance of mutation specific T cell responses in CRC patients. IFN-γ ELISpot analysis with T cells derived from of peripheral blood (PB) and bone marrow (BM) samples of 26 CRC patients. (A) IFN-γ-ELISpot analysis of peripheral blood (PB) derived T cells from a representative (HLA-A*0201 negative) CRC patient carrying the mutation p53 R175H in their primary tumor. Mutated peptide responses were tested against wt peptide responses (black *) and the negative assay control ((-) IgG, gray *). Wt peptide responses were tested against the negative assay control ((-) IgG, gray *) applying unpaired Student’s t-test. The assay was conducted in triplicates. (B) Percentage of patients responding to tested mutated and wt peptides in INFγ-ELISpot analysis. Peptide responses were considered specific in case they were significantly higher than responses towards the negative assay control (IgG, see Figure 1 (A)) in unpaired t-test. (C) Frequency of peptide-specific T cell responses against wt and mutated peptides. Accumulated ELISpot data from blood and BM samples of 26 CRC patients (unpaired t-test). Peptide responses were considered specific in case they were significantly higher than responses towards negative assay control ((-) IgG, see Figure 1 (A)) in unpaired t-test. (D) Distribution of KRAS and TP53 mutations in the tumors and/or metastasis of the CRC patient analyzed previously by ELISpot assay. DNA for exon sequencing of TP53 and KRAS genes was derived from paraffin embedded tissue of primary tumors and metastases of 24 CRC patients. (E) T cell reactivity of patients against wt and mutated peptides correlated with the mutations detected in the patients’ tumors and/or metastasis. panel mut = patients carrying mutations included in the panel of long peptides, other mut = patients carrying TP53 and KRAS mutations different from the mutations included in the panel of long peptides.
