Figure 1.
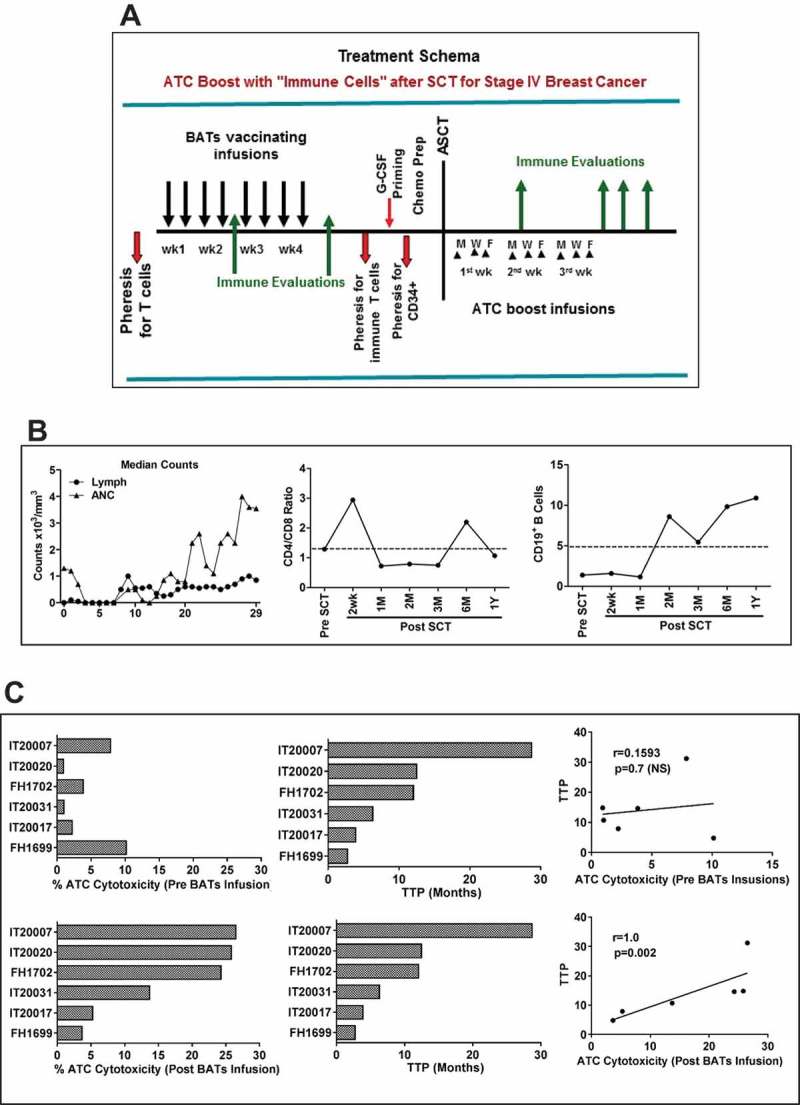
a) Treatment schema shows leukapheresis to obtain T cells for expansion and immunization with BATs. BATs were administered twice weekly for four consecutive weeks. A second leukapheresis was done to obtain immune T cells prior to G-CSF priming for collecting stem cells. PBMC were activated with OKT3 and expanded in IL-2 (100 IU/ml) to generate ATC after 12–14 days of culture. After a 3rd pheresis to collect G-CSF primed CD34+ cells, patients received cyclophosphamide, thiotepa, and carboplatin (CTC) as the preparative regimen for chemosensitive disease and ICE for resistant disease followed by autologous SCT. Immune ATC were infused day + 4 after SCT thrice (n = 2) or twice (n = 4) a week for total 8–15 infusions and immune testing was performed at indicated time points after ATC infusions. b) Shows average daily Lymphocyte (Lymph) and Absolute Neutrophil Counts (ANC) of all 6 evaluable patients (Left panel). Middle and right panels show CD4/CD8 ratio and CD19 + B lymphocytes monitored up to 12 months post SCT (n = 6). C) Shows the bar graph of cytotoxicity (Left) mediated by ATC prior to BATs infusions (top panel) and expanded immune ATC (lower panel) of 6 patients against breast cancer cells (SK-BR-3) measured by the chromium (51Cr) release assay and their corresponding TTP. There were no significant correlation of ATC cytotoxicity prior to BATs infusions with TTP, but there was a significant correlation (p = 0.002) between immune ATC (ATC expanded after BATs infusions) cytotoxicity and TTP (right), suggesting that higher cytotoxicity, may improve progression free survival after BATs infusion contribute to TTP prolongation.
