Figure 7.
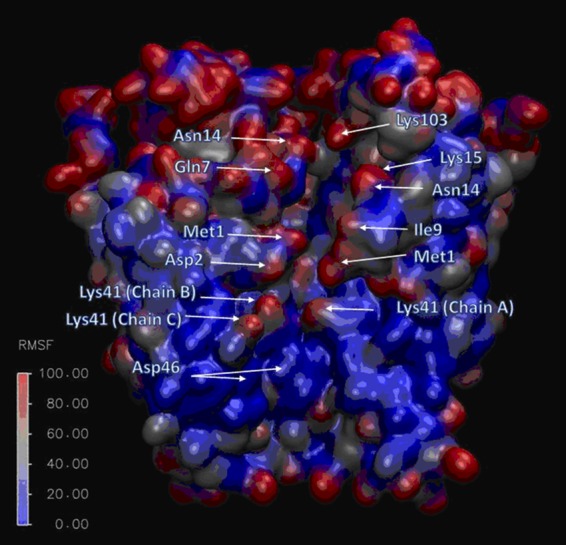
Flexibility of residue side chains lining the connexin pore. Cutaway of Cx26 hemichannel color-coded by per-side-chain RMSF values obtained from umbrella sampling Hamiltonian replica exchange molecular dynamics. Three of the connexin subunits are removed so that the pore can be seen. The cytoplasmic end of the hemichannel is at the top. Most of the residues lining the pore have high RMSF values; in contrast, the ASP46 residue, which corresponds to the peak of the PMF for an impermeant molecule, is relatively rigid (from Luo et al. [2016]). Fig. 7 is reprinted with permission from Biophysical Journal.
